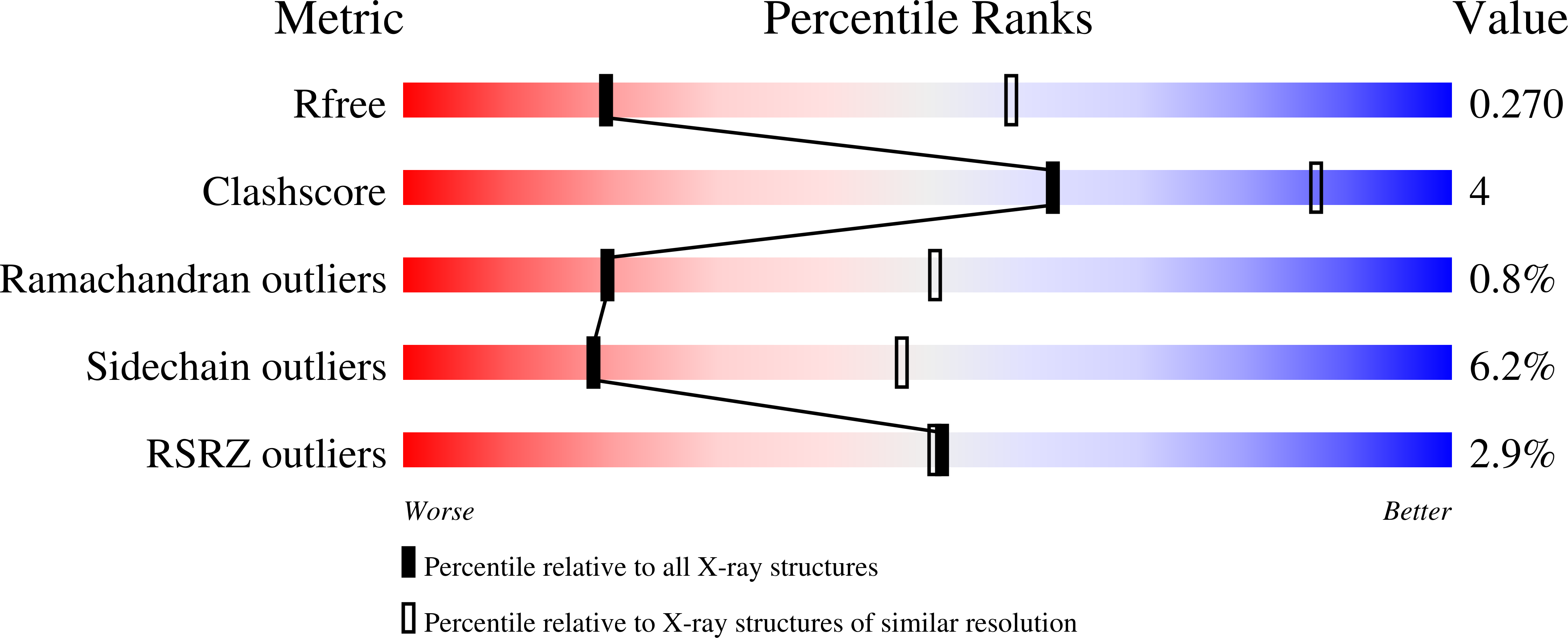
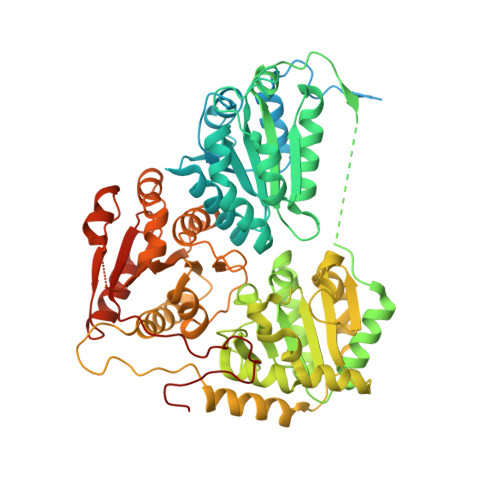
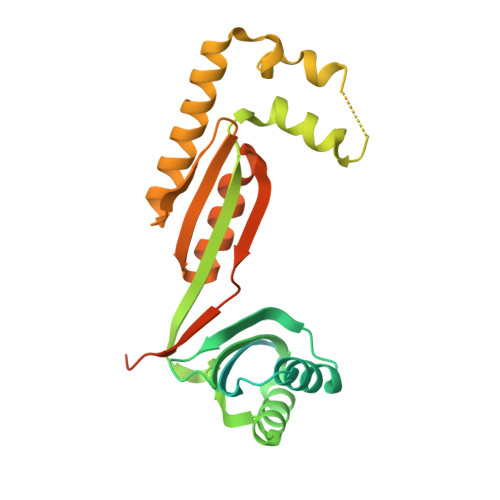
Structures of fungal and plant acetohydroxyacid synthases.
Lonhienne, T., Low, Y.S., Garcia, M.D., Croll, T., Gao, Y., Wang, Q., Brillault, L., Williams, C.M., Fraser, J.A., McGeary, R.P., West, N.P., Landsberg, M.J., Rao, Z., Schenk, G., Guddat, L.W.(2020) Nature 586: 317-321
- PubMed: 32640464
- DOI: https://doi.org/10.1038/s41586-020-2514-3
- Primary Citation of Related Structures:
6U9D, 6U9H, 6VZ8, 6WO1 - PubMed Abstract:
Acetohydroxyacid synthase (AHAS), also known as acetolactate synthase, is a flavin adenine dinucleotide-, thiamine diphosphate- and magnesium-dependent enzyme that catalyses the first step in the biosynthesis of branched-chain amino acids 1 . It is the target for more than 50 commercial herbicides 2 . AHAS requires both catalytic and regulatory subunits for maximal activity and functionality. Here we describe structures of the hexadecameric AHAS complexes of Saccharomyces cerevisiae and dodecameric AHAS complexes of Arabidopsis thaliana. We found that the regulatory subunits of these AHAS complexes form a core to which the catalytic subunit dimers are attached, adopting the shape of a Maltese cross. The structures show how the catalytic and regulatory subunits communicate with each other to provide a pathway for activation and for feedback inhibition by branched-chain amino acids. We also show that the AHAS complex of Mycobacterium tuberculosis adopts a similar structure, thus demonstrating that the overall AHAS architecture is conserved across kingdoms.
Organizational Affiliation:
School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, Queensland, Australia. t.lonhienne@uq.edu.au.