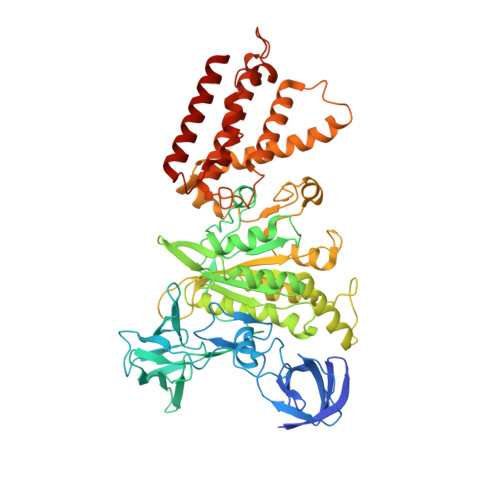
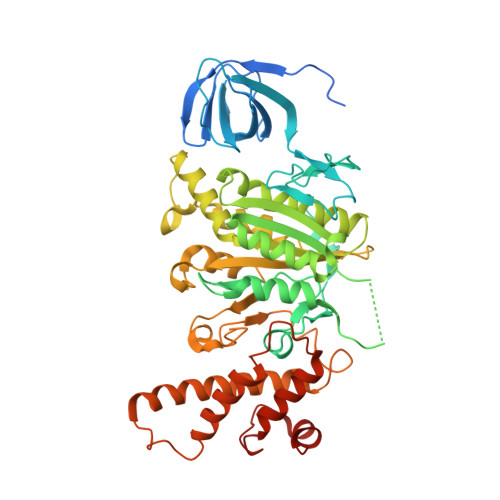
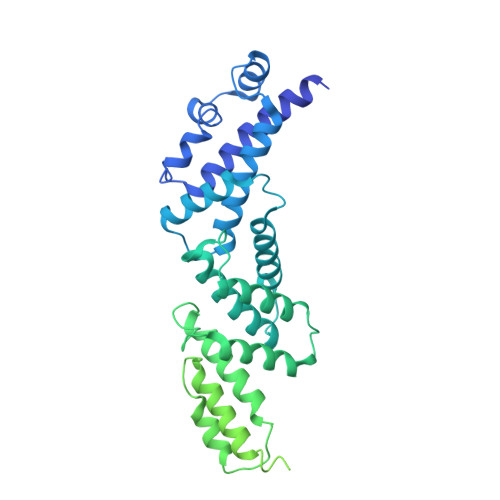
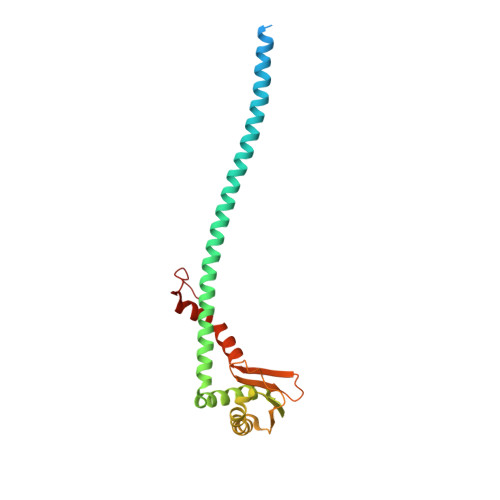
Structures of a Complete Human V-ATPase Reveal Mechanisms of Its Assembly.
Wang, L., Wu, D., Robinson, C.V., Wu, H., Fu, T.M.(2020) Mol Cell 80: 501
- PubMed: 33065002
- DOI: https://doi.org/10.1016/j.molcel.2020.09.029
- Primary Citation of Related Structures:
6WLW, 6WLZ, 6WM2, 6WM3, 6WM4 - PubMed Abstract:
Vesicular- or vacuolar-type adenosine triphosphatases (V-ATPases) are ATP-driven proton pumps comprised of a cytoplasmic V 1 complex for ATP hydrolysis and a membrane-embedded V o complex for proton transfer. They play important roles in acidification of intracellular vesicles, organelles, and the extracellular milieu in eukaryotes. Here, we report cryoelectron microscopy structures of human V-ATPase in three rotational states at up to 2.9-Å resolution. Aided by mass spectrometry, we build all known protein subunits with associated N-linked glycans and identify glycolipids and phospholipids in the V o complex. We define ATP6AP1 as a structural hub for V o complex assembly because it connects to multiple V o subunits and phospholipids in the c-ring. The glycolipids and the glycosylated V o subunits form a luminal glycan coat critical for V-ATPase folding, localization, and stability. This study identifies mechanisms of V-ATPase assembly and biogenesis that rely on the integrated roles of ATP6AP1, glycans, and lipids.
Organizational Affiliation:
Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA; Program in Cellular and Molecular Medicine, Boston Children's Hospital, Boston, MA 02115, USA. Electronic address: wang@hkl.hms.harvard.edu.