Rational Design and Synthesis of Methyl-beta-d-galactomalonyl Phenyl Esters as Potent Galectin-8 N Antagonists.
Patel, B., Kishor, C., Houston, T.A., Shatz-Azoulay, H., Zick, Y., Vinik, Y., Blanchard, H.(2020) J Med Chem 63: 11573-11584
- PubMed: 32809817
- DOI: https://doi.org/10.1021/acs.jmedchem.0c00602
- Primary Citation of Related Structures:
6W4Z - PubMed Abstract:
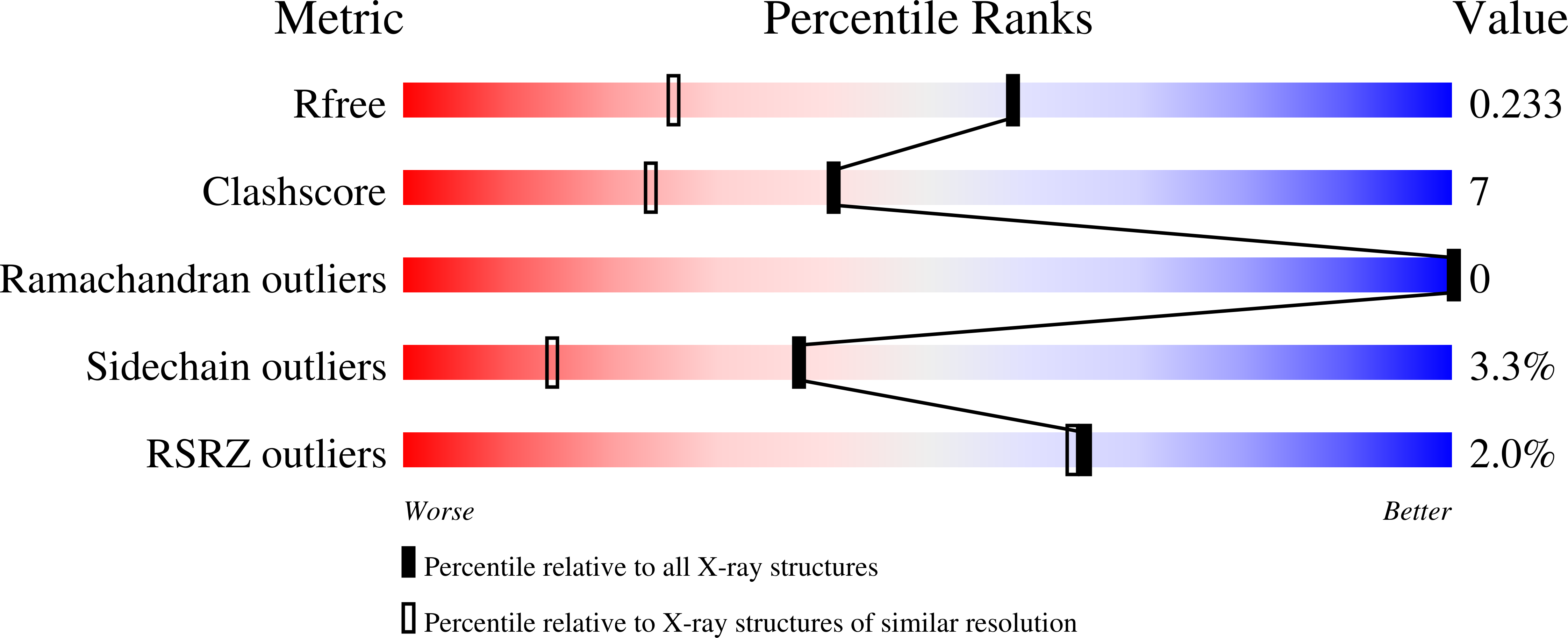
Galectin-8 is a β-galactoside-recognizing protein having an important role in the regulation of bone remodeling and cancer progression and metastasis. Methyl β-d-galactopyranoside malonyl aromatic esters have been designed to target and engage with particular amino acid residues of the galectin-8 N extended carbohydrate-binding site. The chemically synthesized compounds had in vitro binding affinity toward galectin-8 N in the range of 5-33 μM, as evaluated by isothermal titration calorimetry. This affinity directly correlated with the compounds' ability to inhibit galectin-8-induced expression of chemokines and proinflammatory cytokines in the SUM159 breast cancer cell line. X-ray crystallographic structure determination revealed that these monosaccharide-based compounds bind galectin-8 N by engaging its unique arginine (Arg59) and simultaneously cross-linking to another arginine (Arg45) located across the carbohydrate-binding site. This structure-based drug design approach has led to the discovery of novel monosaccharide galactose-based antagonists, with the strongest-binding compound ( K d 5.72 μM) holding 7-fold tighter than the disaccharide lactose.
Organizational Affiliation:
Institute for Glycomics, Griffith University, Gold Coast Campus, Southport, Queensland 4222, Australia.