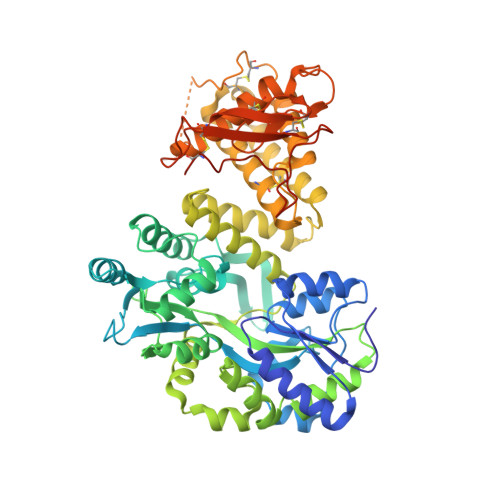
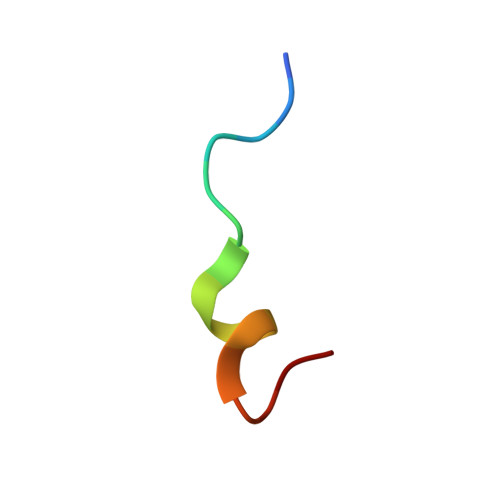
Picomolar Affinity Antagonist and Sustained Signaling Agonist Peptide Ligands for the Adrenomedullin and Calcitonin Gene-Related Peptide Receptors.
Booe, J.M., Warner, M.L., Pioszak, A.A.(2020) Acs Pharmacol Transl Sci 3: 759-772
- PubMed: 32832875
- DOI: https://doi.org/10.1021/acsptsci.0c00031
- Primary Citation of Related Structures:
6V2E - PubMed Abstract:
The calcitonin receptor-like class B G protein-coupled receptor (CLR) mediates adrenomedullin (AM) and calcitonin gene-related peptide (CGRP) functions including vasodilation, cardioprotection, and nociception. Receptor activity-modifying proteins (RAMP1-3) form heterodimers with CLR and determine its peptide ligand selectivity through an unresolved mechanism. The CGRP (RAMP1:CLR) and AM (RAMP2/3:CLR) receptors are proven or promising drug targets, but short AM and CGRP plasma half-lives limit their therapeutic utility. Here, we used synthetic peptide combinatorial library and rational design approaches to probe the ligand selectivity determinants and develop truncated AM and CGRP antagonist variants with receptor extracellular domain binding affinities that were enhanced ∼1000-fold into the low nanomolar range. Receptor binding studies and a high-resolution crystal structure of a novel library-identified AM variant bound to the RAMP2-CLR extracellular domain complex explained the increased affinities and defined roles for AM Lys46 and RAMP modulation of CLR conformation in the ligand selectivity mechanism. In longer AM and CGRP scaffolds that also bind the CLR transmembrane domain, the variants generated picomolar affinity antagonists, one with an estimated 12.5 h CGRP receptor residence time, and sustained signaling agonists "ss-AM" and "ss-CGRP" that exhibited persistent cAMP signaling after ligand washout. Sustained signaling was demonstrated in primary human umbilical vein endothelial cells and the SK-N-MC cell line, which endogenously express AM and CGRP receptors, respectively. This work clarifies the RAMP-modulated CLR ligand selectivity mechanism and provides AM and CGRP variants that are valuable pharmacological tools and may have potential as long-acting therapeutics.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma 73104, United States.