AchievingIn VivoTarget Depletion through the Discovery and Optimization of Benzimidazolone BCL6 Degraders.
Bellenie, B.R., Cheung, K.J., Varela, A., Pierrat, O.A., Collie, G.W., Box, G.M., Bright, M.D., Gowan, S., Hayes, A., Rodrigues, M.J., Shetty, K.N., Carter, M., Davis, O.A., Henley, A.T., Innocenti, P., Johnson, L.D., Liu, M., de Klerk, S., Le Bihan, Y.V., Lloyd, M.G., McAndrew, P.C., Shehu, E., Talbot, R., Woodward, H.L., Burke, R., Kirkin, V., van Montfort, R.L.M., Raynaud, F.I., Rossanese, O.W., Hoelder, S.(2020) J Med Chem 63: 4047-4068
- PubMed: 32275432
- DOI: https://doi.org/10.1021/acs.jmedchem.9b02076
- Primary Citation of Related Structures:
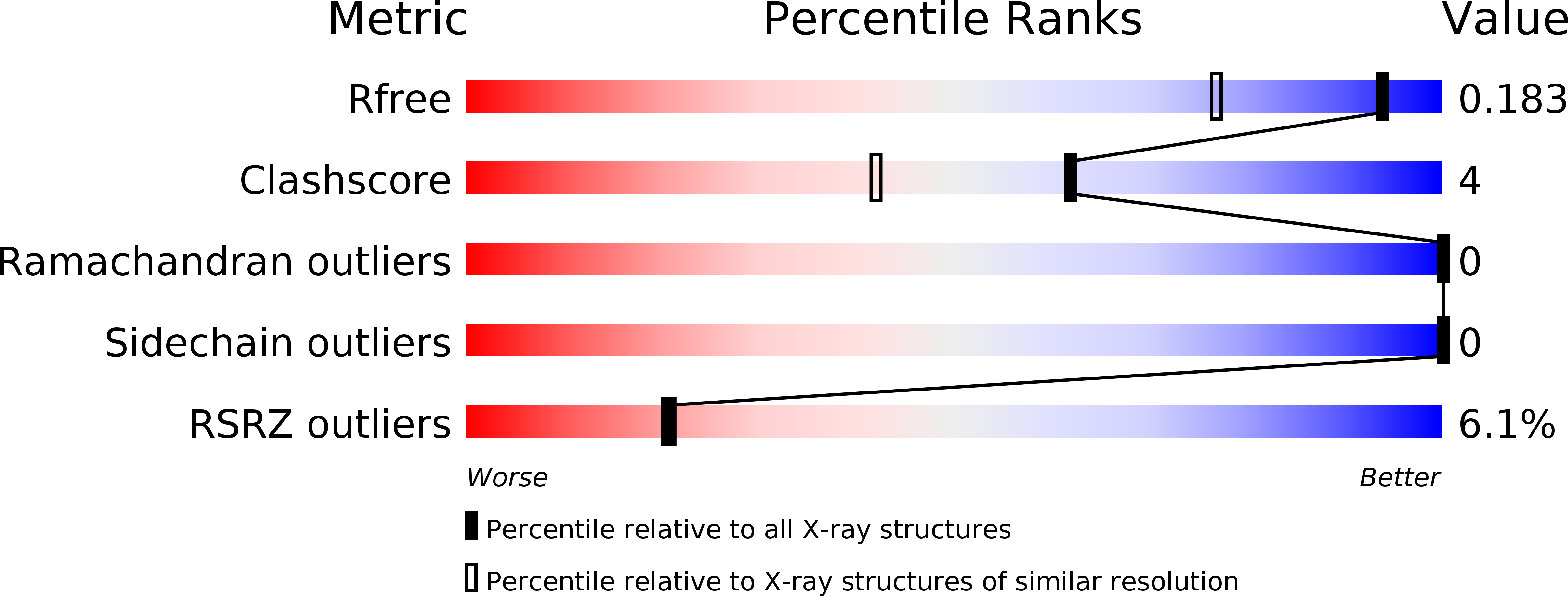
6TOF, 6TOG, 6TOH, 6TOI, 6TOJ, 6TOK, 6TOL, 6TOM, 6TON, 6TOO - PubMed Abstract:
Deregulation of the transcriptional repressor BCL6 enables tumorigenesis of germinal center B-cells, and hence BCL6 has been proposed as a therapeutic target for the treatment of diffuse large B-cell lymphoma (DLBCL). Herein we report the discovery of a series of benzimidazolone inhibitors of the protein-protein interaction between BCL6 and its co-repressors. A subset of these inhibitors were found to cause rapid degradation of BCL6, and optimization of pharmacokinetic properties led to the discovery of 5-((5-chloro-2-((3 R ,5 S )-4,4-difluoro-3,5-dimethylpiperidin-1-yl)pyrimidin-4-yl)amino)-3-(3-hydroxy-3-methylbutyl)-1-methyl-1,3-dihydro-2 H -benzo[ d ]imidazol-2-one (CCT369260), which reduces BCL6 levels in a lymphoma xenograft mouse model following oral dosing.