A Targeted Protein Degradation Cell-Based Screening for Nanobodies Selective toward the Cellular RHOB GTP-Bound Conformation.
Bery, N., Keller, L., Soulie, M., Gence, R., Iscache, A.L., Cherier, J., Cabantous, S., Sordet, O., Lajoie-Mazenc, I., Pedelacq, J.D., Favre, G., Olichon, A.(2019) Cell Chem Biol 26: 1544
- PubMed: 31522999
- DOI: https://doi.org/10.1016/j.chembiol.2019.08.009
- Primary Citation of Related Structures:
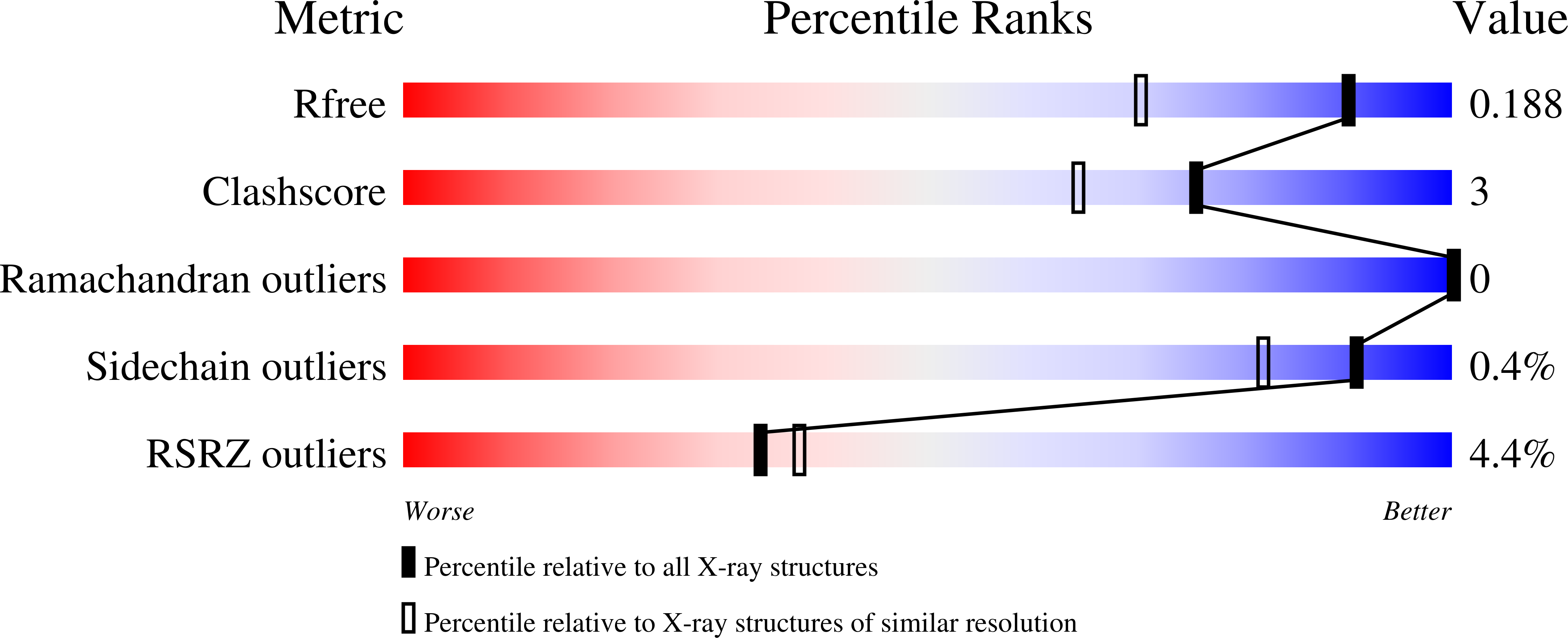
6HXU, 6SGE - PubMed Abstract:
The selective downregulation of activated intracellular proteins is a key challenge in cell biology. RHO small GTPases switch between a guanosine diphosphate (GDP)-bound and a guanosine triphosphate (GTP)-bound state that drives downstream signaling. At present, no tool is available to study endogenous RHO-GTPinduced conformational changes in live cells. Here, we established a cell-based screen to selectively degrade RHOB-GTP using F-box-intracellular single-domain antibody fusion. We identified one intracellular antibody (intrabody) that shows selective targeting of endogenous RHOB-GTP mediated by interactions between the CDR3 loop of the domain antibody and the GTP-binding pocket of RHOB. Our results suggest that, while RHOB is highly regulated at the expression level, only the GTP-bound pool, but not its global expression, mediates RHOB functions in genomic instability and in cell invasion. The F-box/intrabody-targeted protein degradation represents a unique approach to knock down the active form of small GTPases or other proteins with multiple cellular activities.
Organizational Affiliation:
Centre de Recherche en Cancérologie de Toulouse (CRCT), INSERM, Université de Toulouse, CNRS, UPS, Toulouse, France.