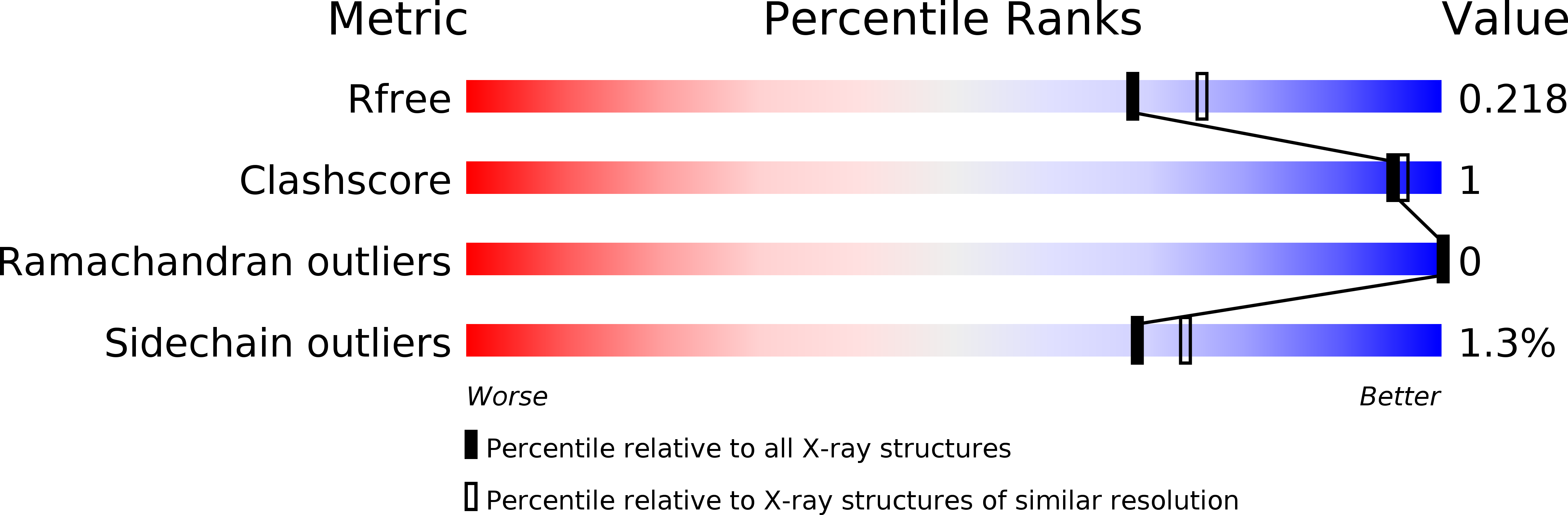
Metal Positions and Translocation Pathways of the Dodecameric Ferritin-like Protein Dps.
Zeth, K., Sancho-Vaello, E., Okuda, M.(2019) Inorg Chem 58: 11351-11363
- PubMed: 31433627
- DOI: https://doi.org/10.1021/acs.inorgchem.9b00301
- Primary Citation of Related Structures:
6HUI, 6HV1, 6HVQ, 6HX2, 6SEV - PubMed Abstract:
Iron storage in biology is carried out by cage-shaped proteins of the ferritin superfamily, one of which is the dodecameric protein Dps. In Dps, four distinct steps lead to the formation of metal nanoparticles: attraction of ion-aquo complexes to the protein matrix, passage of these complexes through translocation pores, oxidation of these complexes at ferroxidase centers, and, ultimately, nanoparticle formation. In this study, we investigated Dps from Listeria innocua to structurally characterize these steps for Co 2+ , Zn 2+ , and La 3+ ions. The structures reveal that differences in their ion coordination chemistry determine alternative metal ion-binding sites on the areas of the surface surrounding the translocation pore that captures nine La 3+ , three Co 2+ , or three Zn 2+ ions as aquo clusters and passes them on for translocation. Inside these pores, ion-selective conformational changes at key residues occur before a gating residue to actively move ions through the constriction zone. Ions upstream of the Asp130 gate residue are typically hydrated, while ions downstream directly interact with the protein matrix. Inside the cavity, ions move along negatively charged residues to the ferroxidase center, where seven main residues adapt to the three different ions by dynamically changing their conformations. In total, we observed more than 20 metal-binding sites per Dps monomer, which clearly highlights the metal-binding capacity of this protein family. Collectively, our results provide a detailed structural description of the preparative steps for amino acid-assisted biomineralization in Dps proteins, demonstrating unexpected protein matrix plasticity.
Organizational Affiliation:
Roskilde University , Department of Science and Environment , Universitetsvej 1 , 4000 Roskilde , Denmark.