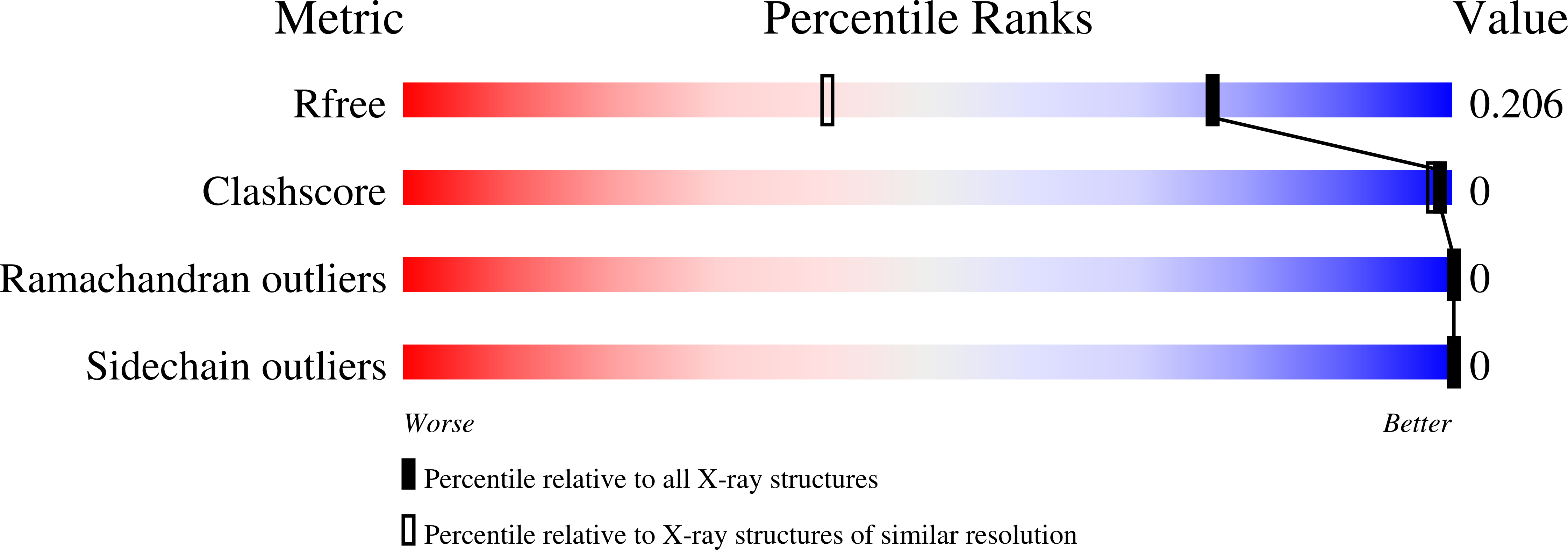4-Acyl Pyrroles as Dual BET-BRD7/9 Bromodomain Inhibitors Address BETi Insensitive Human Cancer Cell Lines.
Hugle, M., Regenass, P., Warstat, R., Hau, M., Schmidtkunz, K., Lucas, X., Wohlwend, D., Einsle, O., Jung, M., Breit, B., Gunther, S.(2020) J Med Chem 63: 15603-15620
- PubMed: 33275431
- DOI: https://doi.org/10.1021/acs.jmedchem.0c00478
- Primary Citation of Related Structures:
6RWJ, 6S4B, 6S6K, 6SA2, 6SA3, 6SAH, 6SAJ, 6SB8 - PubMed Abstract:
Various malignant human diseases show disturbed signaling pathways due to increased activity of proteins within the epigenetic machinery. Recently, various novel inhibitors for epigenetic regulation have been introduced which promise a great therapeutic benefit. Inhibitors for the bromo- and extra-terminal domain (BET) family were of particular interest after inhibitors had shown a strong antiproliferative effect. More recently, the focus has increasingly shifted to bromodomains (BDs) outside the BET family. Based on previously developed inhibitors, we have optimized a small series of 4-acyl pyrroles, which we further analyzed by ITC, X-ray crystallography, selectivity studies, the NCI60 cell-panel, and GI 50 determinations for several cancer cell lines. The inhibitors address both, BET and BRD7/9 BDs, with very high affinity and show a strong antiproliferative effect on various cancer cell lines that could not be observed for BD family selective inhibitors. Furthermore, a synergistic effect on breast cancer (MCF-7) and melanoma (SK-MEL-5) was proven.
Organizational Affiliation:
Institut für Pharmazeutische Wissenschaften, Albert-Ludwigs-Universität Freiburg, Hermann-Herder-Str. 8, D-79104 Freiburg, Germany.