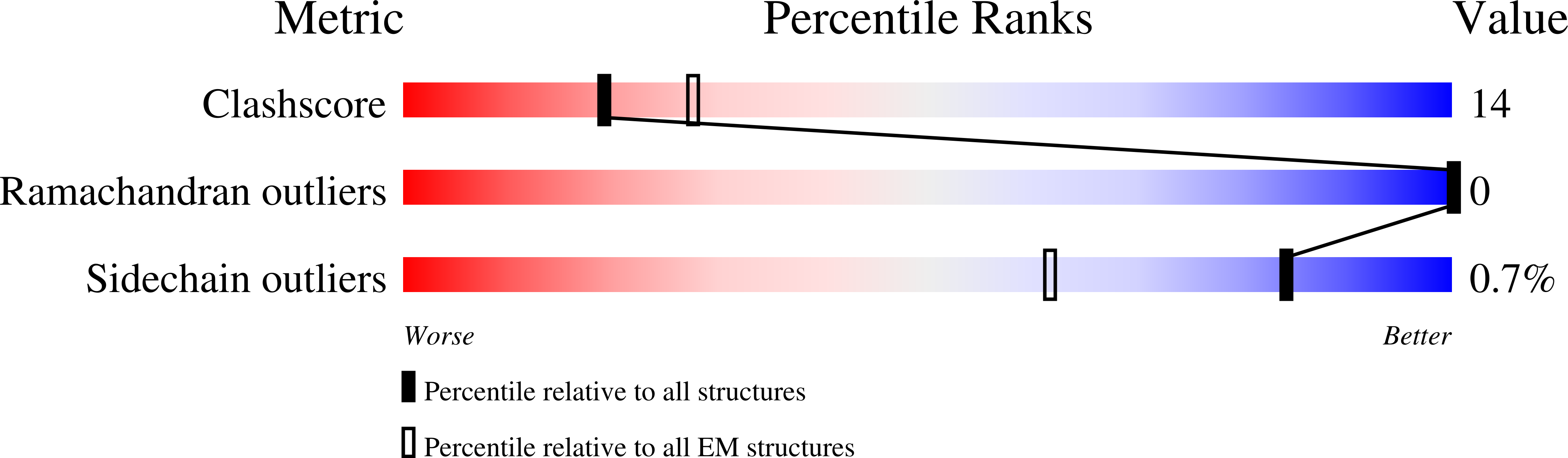Molybdate pumping into the molybdenum storage protein via an ATP-powered piercing mechanism.
Brunle, S., Eisinger, M.L., Poppe, J., Mills, D.J., Langer, J.D., Vonck, J., Ermler, U.(2019) Proc Natl Acad Sci U S A
- PubMed: 31811022
- DOI: https://doi.org/10.1073/pnas.1913031116
- Primary Citation of Related Structures:
6RIS, 6RJ4, 6RKD, 6RKE - PubMed Abstract:
The molybdenum storage protein (MoSto) deposits large amounts of molybdenum as polyoxomolybdate clusters in a heterohexameric (αβ) 3 cage-like protein complex under ATP consumption. Here, we suggest a unique mechanism for the ATP-powered molybdate pumping process based on X-ray crystallography, cryoelectron microscopy, hydrogen-deuterium exchange mass spectrometry, and mutational studies of MoSto from Azotobacter vinelandii . First, we show that molybdate, ATP, and Mg 2+ consecutively bind into the open ATP-binding groove of the β-subunit, which thereafter becomes tightly locked by fixing the previously disordered N-terminal arm of the α-subunit over the β-ATP. Next, we propose a nucleophilic attack of molybdate onto the γ-phosphate of β-ATP, analogous to the similar reaction of the structurally related UMP kinase. The formed instable phosphoric-molybdic anhydride becomes immediately hydrolyzed and, according to the current data, the released and accelerated molybdate is pressed through the cage wall, presumably by turning aside the Metβ149 side chain. A structural comparison between MoSto and UMP kinase provides valuable insight into how an enzyme is converted into a molecular machine during evolution. The postulated direct conversion of chemical energy into kinetic energy via an activating molybdate kinase and an exothermic pyrophosphatase reaction to overcome a proteinous barrier represents a novelty in ATP-fueled biochemistry, because normally, ATP hydrolysis initiates large-scale conformational changes to drive a distant process.
Organizational Affiliation:
Department of Molecular Membrane Biology, Max Planck Institute of Biophysics, 60438 Frankfurt am Main, Germany.