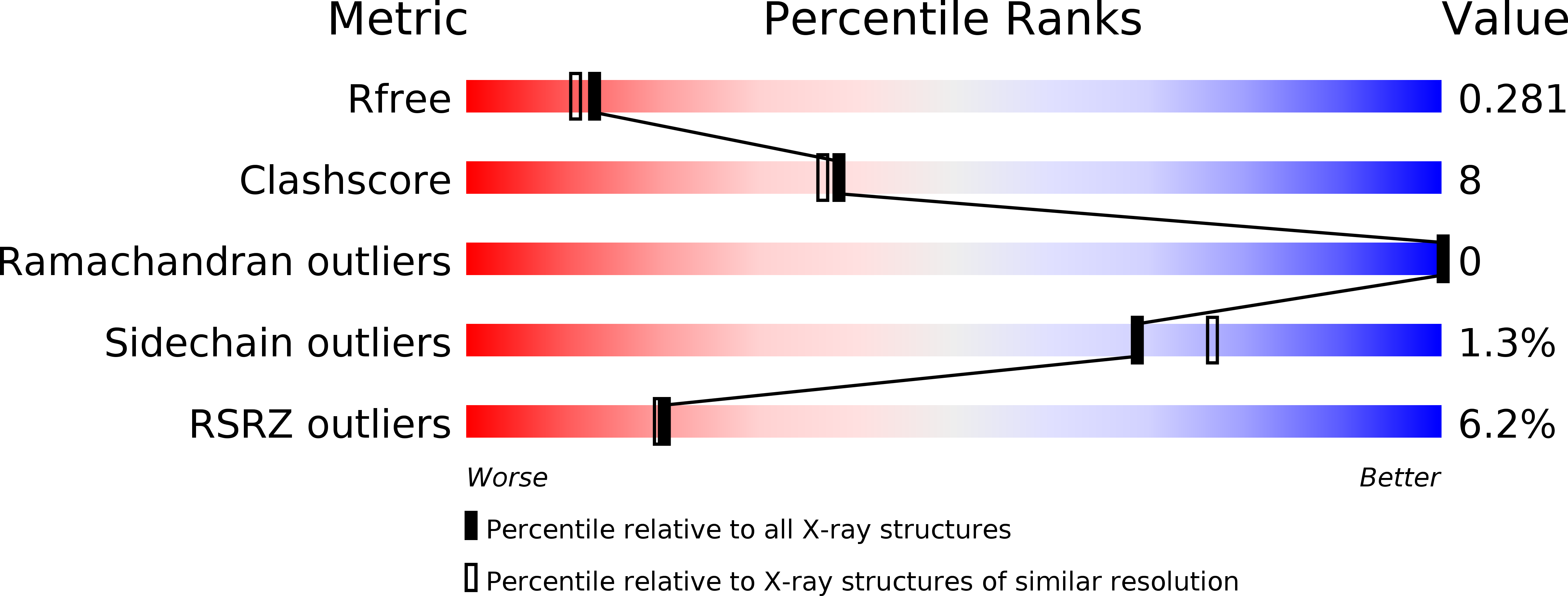
Identification and Characterization of Mutations in Ubiquitin Required for Non-covalent Dimer Formation.
Gabrielsen, M., Buetow, L., Kowalczyk, D., Zhang, W., Sidhu, S.S., Huang, D.T.(2019) Structure 27: 1452-1459.e4
- PubMed: 31303481
- DOI: https://doi.org/10.1016/j.str.2019.06.008
- Primary Citation of Related Structures:
6QK9 - PubMed Abstract:
Ubiquitin (Ub) is a small protein that post-translationally modifies a variety of substrates in eukaryotic cells to modulate substrate function. The ability of Ub to interact with numerous protein domains makes Ub an attractive scaffold for engineering ubiquitin variants (UbVs) with high target specificity. Previously, we identified a UbV that formed a non-covalent stable dimer via a β-strand exchange, and in the current work we identified and characterized the minimal substitutions in the primary sequence of Ub required to form a higher ordered complex. Using solution angle scattering and X-ray crystallography, we show that a single substitution of residue Gly10 to either Ala or Val is sufficient to convert Ub from a monomer to a dimer. We also investigate contributions to dimer formation by the residues in the surrounding sequence. These results can be used to develop next-generation phage-display libraries of UbVs to engineer new interfaces for protein recognition.
Organizational Affiliation:
Cancer Research UK Beatson Institute, Institute of Cancer Sciences, University of Glasgow, Garscube Estate, Switchback Road, Glasgow G61 1BD, UK.