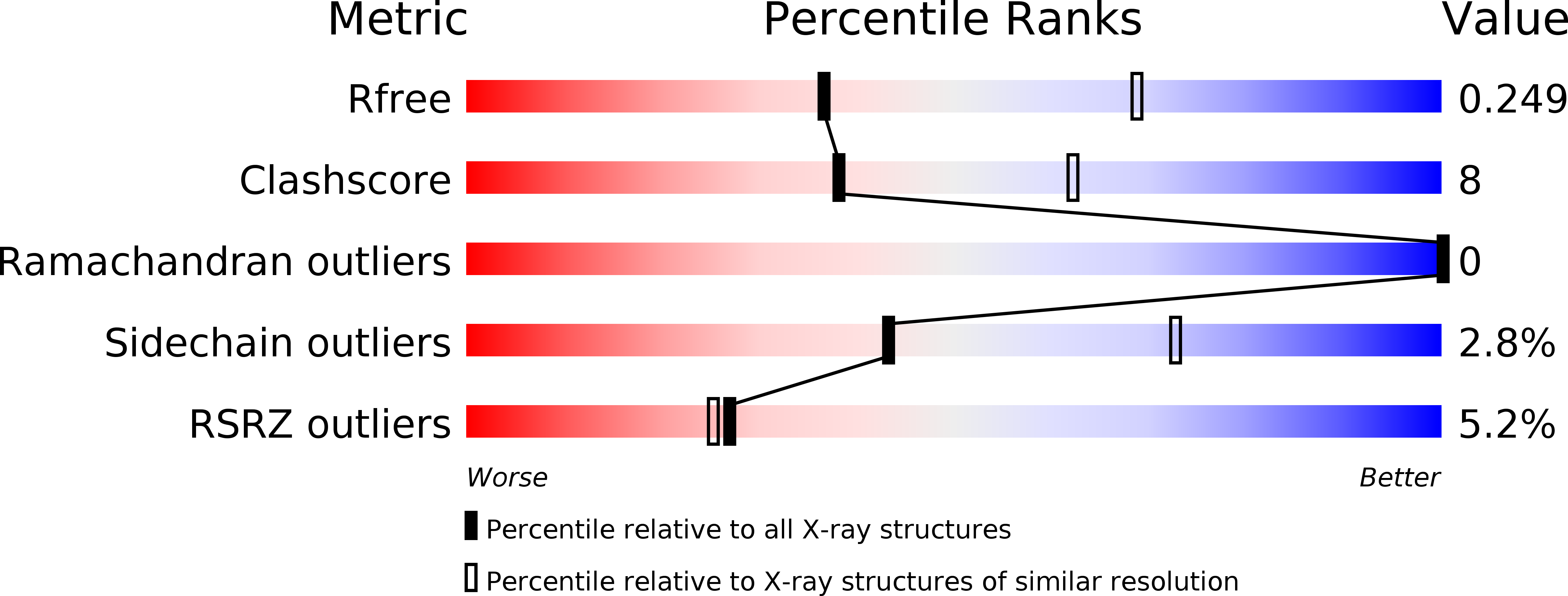
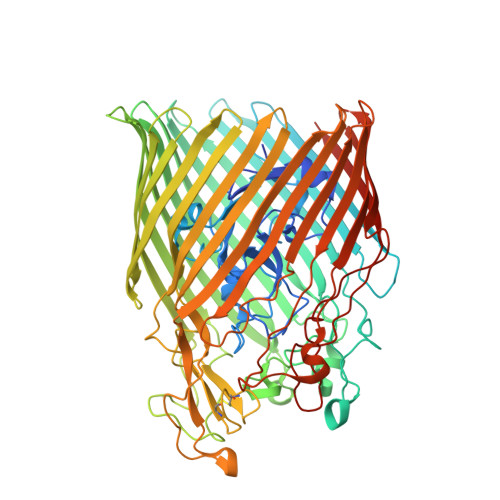
The complex of ferric-enterobactin with its transporter from Pseudomonas aeruginosa suggests a two-site model.
Moynie, L., Milenkovic, S., Mislin, G.L.A., Gasser, V., Malloci, G., Baco, E., McCaughan, R.P., Page, M.G.P., Schalk, I.J., Ceccarelli, M., Naismith, J.H.(2019) Nat Commun 10: 3673-3673
- PubMed: 31413254
- DOI: https://doi.org/10.1038/s41467-019-11508-y
- Primary Citation of Related Structures:
5M9B, 5MZS, 5NC4, 5NR2, 5OUT, 6I2J, 6Q5E, 6R1F - PubMed Abstract:
Bacteria use small molecules called siderophores to scavenge iron. Siderophore-Fe 3+ complexes are recognised by outer-membrane transporters and imported into the periplasm in a process dependent on the inner-membrane protein TonB. The siderophore enterobactin is secreted by members of the family Enterobacteriaceae, but many other bacteria including Pseudomonas species can use it. Here, we show that the Pseudomonas transporter PfeA recognises enterobactin using extracellular loops distant from the pore. The relevance of this site is supported by in vivo and in vitro analyses. We suggest there is a second binding site deeper inside the structure and propose that correlated changes in hydrogen bonds link binding-induced structural re-arrangements to the structural adjustment of the periplasmic TonB-binding motif.
Organizational Affiliation:
Division of Structural Biology, Wellcome Trust Centre of Human Genomics, 7 Roosevelt Drive, Oxford, OX3 7BN, UK.