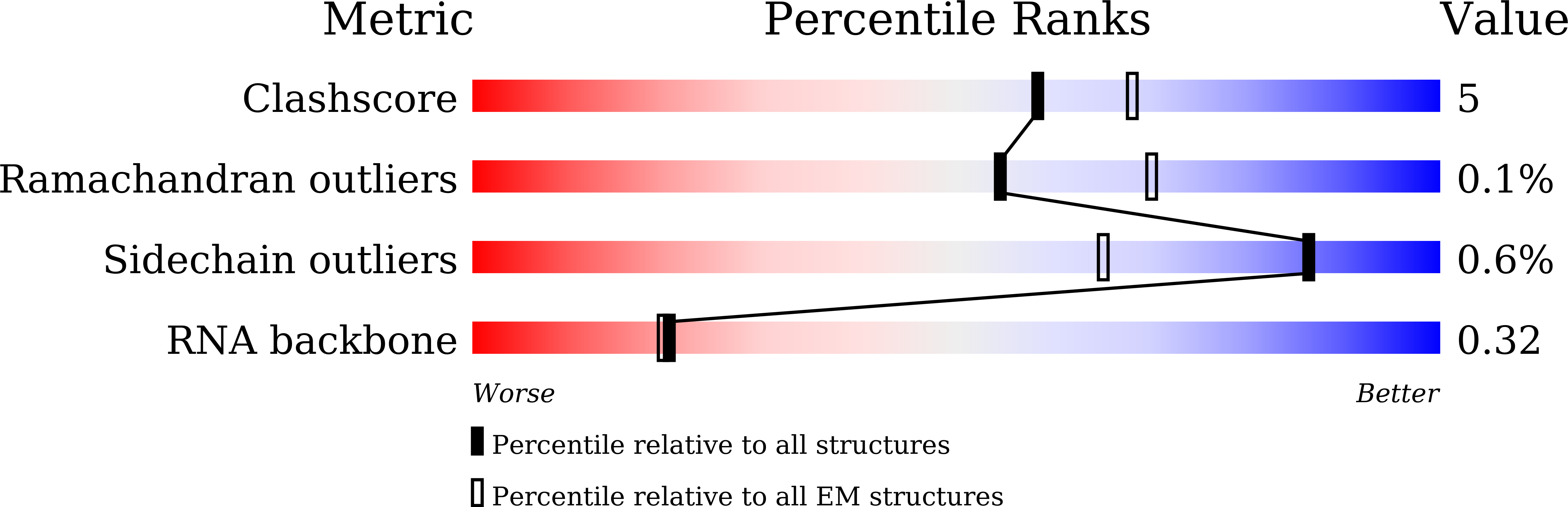
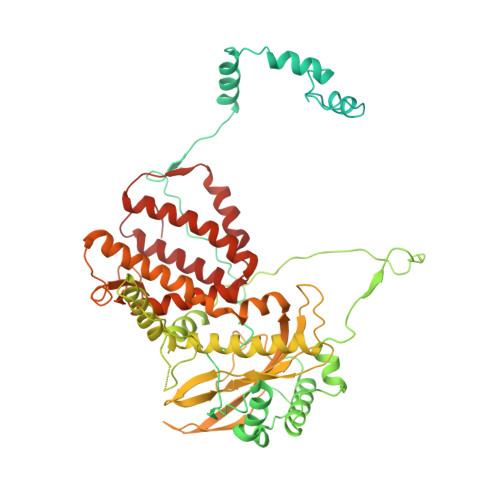
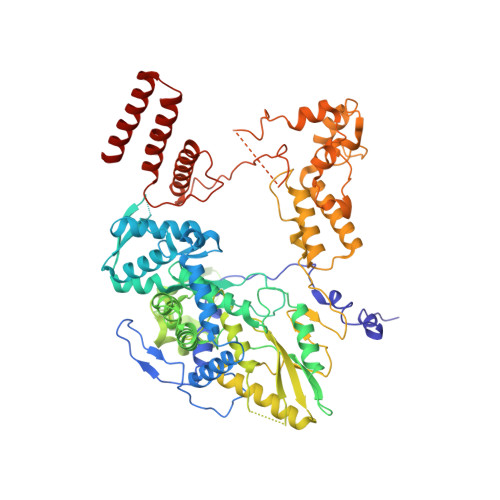
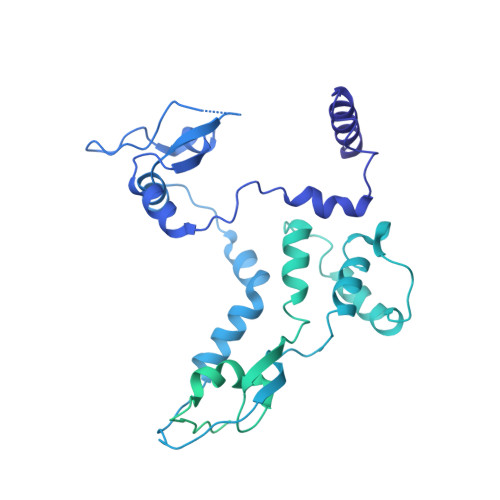
Structure of influenza D virus polymerase bound to cRNA promoter in Mode A conformation
Peng, Q., Liu, Y., Peng, R., Wang, M., Yang, W., Song, H., Chen, Y., Liu, S., Han, M., Zhang, X., Wang, P., Yan, J., Zhang, B., Qi, J., Deng, T., Gao, G.F., Shi, Y.(2019) Nat Microbiol
- PubMed: 31209309
- DOI: https://doi.org/10.1038/s41564-019-0487-5
- Primary Citation of Related Structures:
6KUJ, 6KUK, 6KUP, 6KUR, 6KUT, 6KUV, 6KV5 - PubMed Abstract:
The influenza virus polymerase uses capped RNA primers to initiate transcription, and a combination of terminal and internal de novo initiations for the two-step replication process by binding the conserved viral genomic RNA (vRNA) or complementary RNA (cRNA) promoter. Here, we determined the apo and promoter-bound influenza D polymerase structures using cryo-electron microscopy and found the polymerase has an evolutionarily conserved stable core structure with inherently flexible peripheral domains. Strikingly, two conformations (mode A and B) of the vRNA promoter were observed where the 3'-vRNA end can bind at two different sites, whereas the cRNA promoter only binds in the mode B conformation. Functional studies confirmed the critical role of the mode B conformation for vRNA synthesis via the intermediate cRNA but not for cRNA production, which is mainly regulated by the mode A conformation. Both conformations participate in the regulation of the transcription process. This work advances our understanding of the regulatory mechanisms for the synthesis of different RNA species by influenza virus polymerase and opens new opportunities for antiviral drug design.
Organizational Affiliation:
CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China.