Molecular Basis for PI(3,5)P2Recognition by SNX11, a Protein Involved in Lysosomal Degradation and Endosome Homeostasis Regulation.
Xu, T., Gan, Q., Wu, B., Yin, M., Xu, J., Shu, X., Liu, J.(2020) J Mol Biol 432: 4750-4761
- PubMed: 32561432
- DOI: https://doi.org/10.1016/j.jmb.2020.06.010
- Primary Citation of Related Structures:
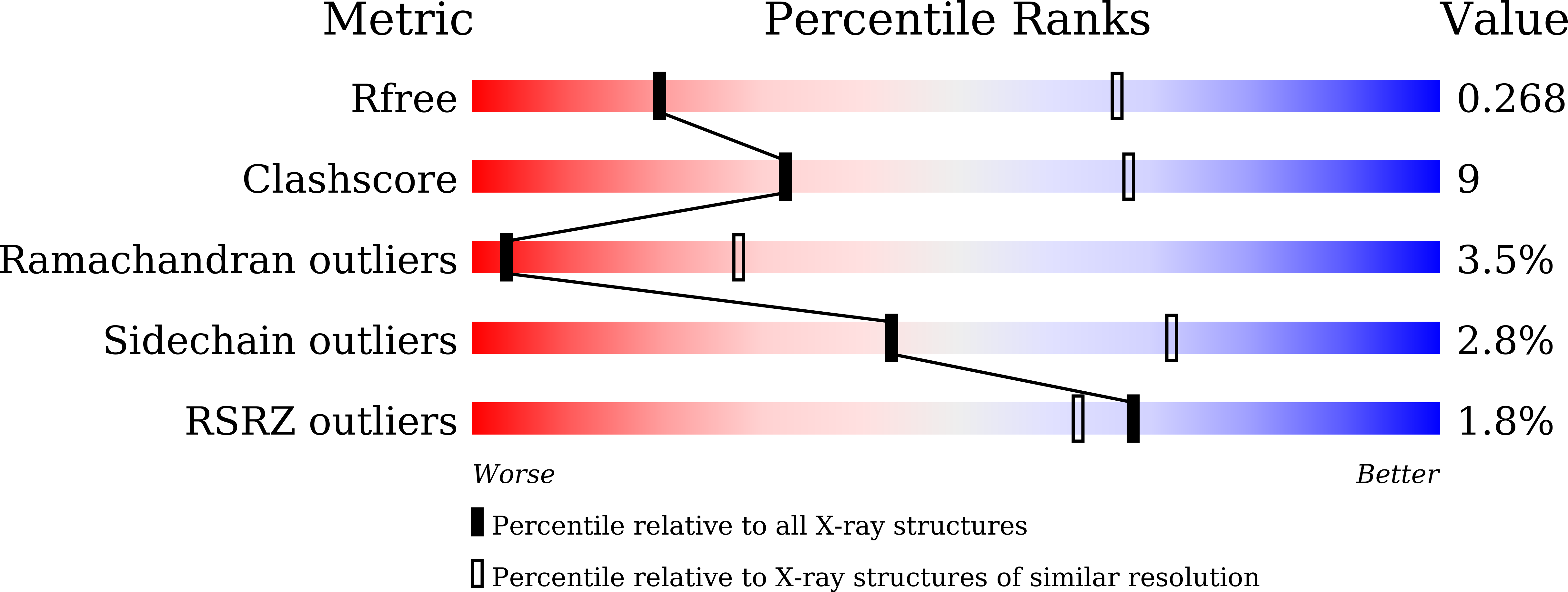
6KOI, 6KOJ, 6KOK - PubMed Abstract:
Phosphatidylinositol 3,5-bisphosphate (PI(3,5)P 2 ) is an essential phosphoinositide required for endosome homeostasis and sorting for lysosomal degradation; however, the underlying mechanisms, especially in mammals, remain elusive or unexplored. Here we determined a structure of PI(3,5)P 2 bound to Sorting Nexin 11 (SNX11) with an opened PPII-C loop. We also obtained an SNX11 structure with its PPII-C in "closed" form that serves as a potential PI3P-binding model. In addition, our results reveal that SNX11 can interact with the V1D subunit of vacuolar H + -ATPase (V-ATPase), which provides a link between PI(3,5)P 2 and human V-ATPase and further evidence for their roles in the endosome homeostasis regulation. Lastly, a new apo-form structure of SNX11, combined with molecular dynamics (MD) studies, indicates that the α5 helix can unfold from the PX domain of SNX11 when targeting the membrane or interacting with its partner. Taken together, these findings identify a novel PI(3,5)P 2 effector, which will shed light on the PIs recognizing mechanism and the understanding of the downstream sorting events triggered by different PI binding.
Organizational Affiliation:
State Key Laboratory of Respiratory Disease, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou 510530, China; Guangdong Provincial Key Laboratory of Biocomputing, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences, Guangzhou 510530, China. Electronic address: xu_tingting@gibh.ac.cn.