Complex of GGTaseIII, farnesyl-Ykt6 (C-terminal methylated), and GGPP
Goto-Ito, S., Shirakawa, R., Fukai, S.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein prenyltransferase alpha subunit repeat-containing protein 1 | 327 | Homo sapiens | Mutation(s): 0 Gene Names: PTAR1 | 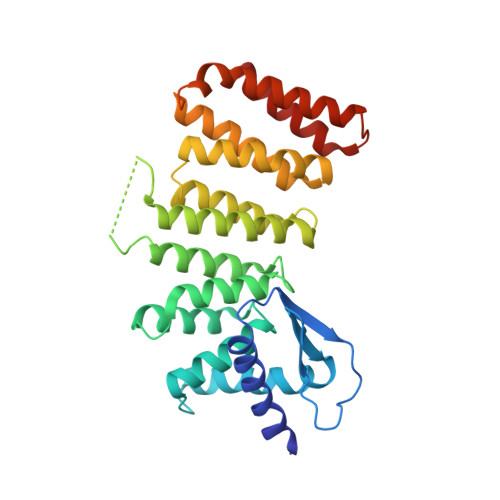 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q7Z6K3 (Homo sapiens) Explore Q7Z6K3 Go to UniProtKB: Q7Z6K3 | |||||
PHAROS: Q7Z6K3 GTEx: ENSG00000188647 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q7Z6K3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Geranylgeranyl transferase type-2 subunit beta | 336 | Homo sapiens | Mutation(s): 0 Gene Names: RABGGTB, GGTB EC: 2.5.1.60 | 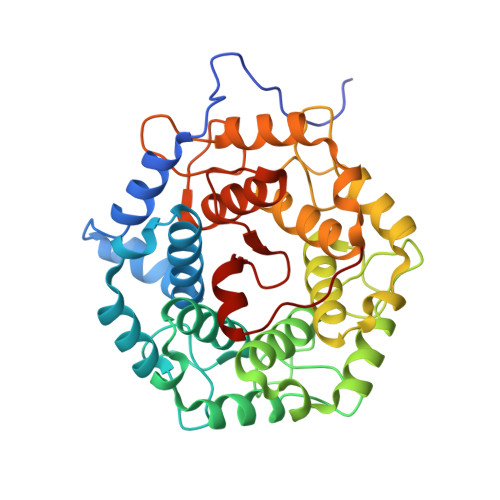 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P53611 (Homo sapiens) Explore P53611 Go to UniProtKB: P53611 | |||||
PHAROS: P53611 GTEx: ENSG00000137955 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P53611 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Synaptobrevin homolog YKT6 | 195 | Homo sapiens | Mutation(s): 0 Gene Names: YKT6 EC: 2.3.1 | 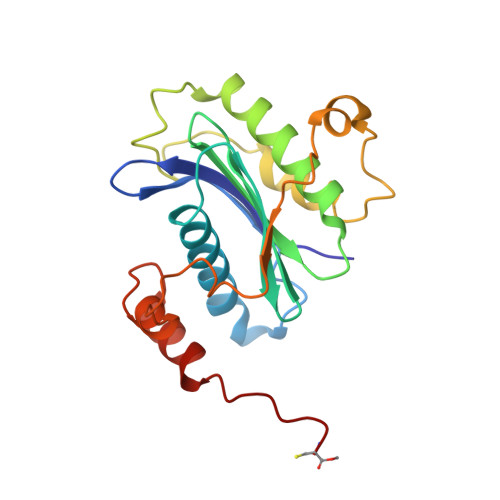 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15498 (Homo sapiens) Explore O15498 Go to UniProtKB: O15498 | |||||
PHAROS: O15498 GTEx: ENSG00000106636 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15498 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GER (Subject of Investigation/LOI) Query on GER | F [auth B] | GERAN-8-YL GERAN C20 H34 HSOYJGBJQAKCNA-CAIKYXSQSA-N |  | ||
| FAR (Subject of Investigation/LOI) Query on FAR | E [auth B] | FARNESYL C15 H26 JXBSHSBNOVLGHF-BUJBXKITSA-N |  | ||
| DPO Query on DPO | G [auth B] | DIPHOSPHATE O7 P2 XPPKVPWEQAFLFU-UHFFFAOYSA-J |  | ||
| TLA Query on TLA | D [auth B] | L(+)-TARTARIC ACID C4 H6 O6 FEWJPZIEWOKRBE-JCYAYHJZSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CMT Query on CMT | C | L-PEPTIDE LINKING | C4 H9 N O2 S |  | CYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 119.415 | α = 90 |
| b = 119.415 | β = 90 |
| c = 210.649 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Japan Science and Technology | Japan | CREST JPMJCR12M5 |
| Japan Society for the Promotion of Science | Japan | KAKENHI 16K08574 |
| Japan Society for the Promotion of Science | Japan | KAKENHI 16H05148 |