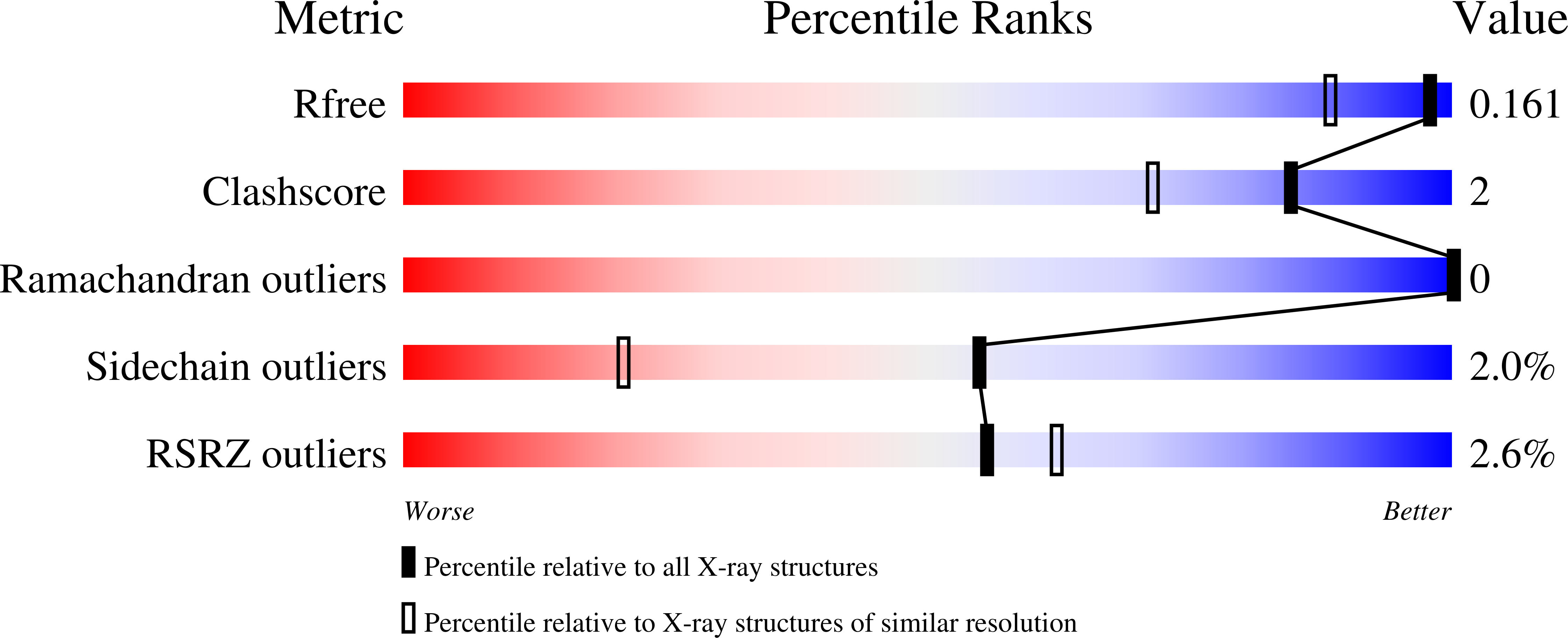
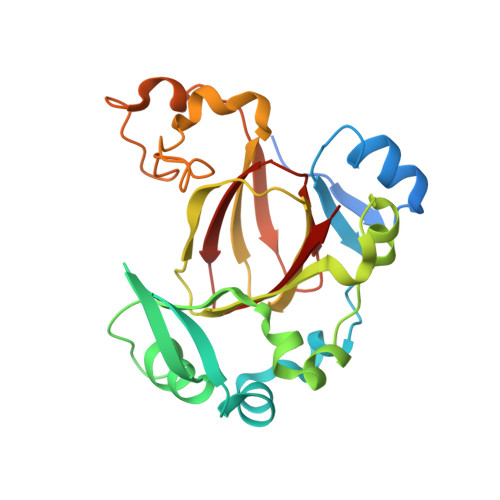
Structural analysis of the 2-oxoglutarate binding site of the circadian rhythm linked oxygenase JMJD5.
Islam, M.S., Markoulides, M., Chowdhury, R., Schofield, C.J.(2022) Sci Rep 12: 20680-20680
- PubMed: 36450832
- DOI: https://doi.org/10.1038/s41598-022-24154-0
- Primary Citation of Related Structures:
6I9L, 6I9M, 6I9N, 7UQ3 - PubMed Abstract:
JmjC (Jumonji-C) domain-containing 5 (JMJD5) plays important roles in circadian regulation in plants and humans and is involved in embryonic development and cell proliferation. JMJD5 is a 2-oxoglutarate (2OG) and Fe(II) dependent oxygenase of the JmjC subfamily, which includes histone N ε -methyl lysine-demethylases (KDMs) and hydroxylases catalysing formation of stable alcohol products. JMJD5 is reported to have KDM activity, but has been shown to catalyse C-3 hydroxylation of arginine residues in sequences from human regulator of chromosome condensation domain-containing protein 1 (RCCD1) and ribosomal protein S6 (RPS6) in vitro. We report crystallographic analyses of human JMJD5 complexed with 2OG analogues, including the widely used hypoxia mimic pyridine-2,4-dicarboxylate, both D- and L-enantiomers of the oncometabolite 2-hydroxyglutarate, and a cyclic N-hydroxyimide. The results support the assignment of JMJD5 as a protein hydroxylase and reveal JMJD5 has an unusually compact 2OG binding pocket suitable for exploitation in development of selective inhibitors. They will be useful in the development of chemical probes to investigate the physiologically relevant roles of JMJD5 in circadian rhythm and development and explore its potential as a medicinal chemistry target.
Organizational Affiliation:
Chemistry Research Laboratory, Department of Chemistry and the Ineos Oxford Institute for Antimicrobial Research, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK.