Type 9 secretion system structures reveal a new protein transport mechanism.
Lauber, F., Deme, J.C., Lea, S.M., Berks, B.C.(2018) Nature 564: 77-82
- PubMed: 30405243
- DOI: https://doi.org/10.1038/s41586-018-0693-y
- Primary Citation of Related Structures:
6H3I, 6H3J - PubMed Abstract:
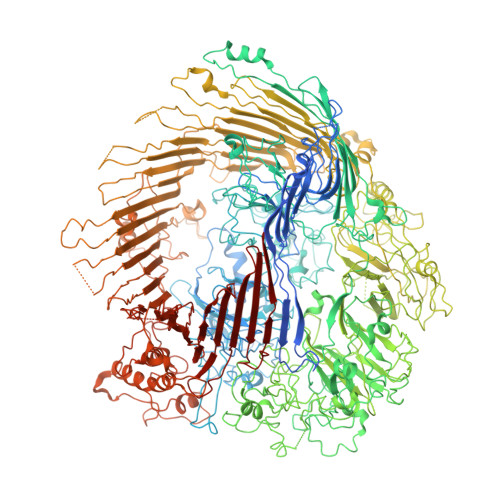
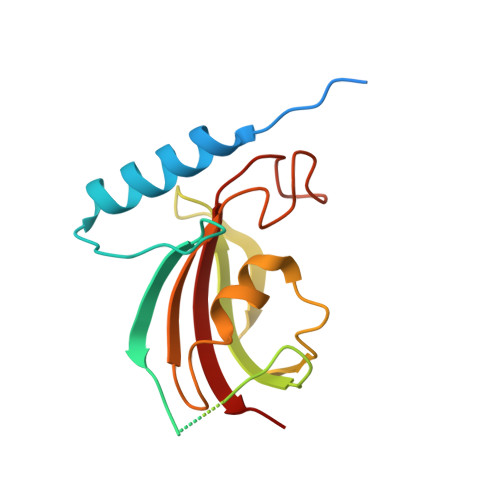
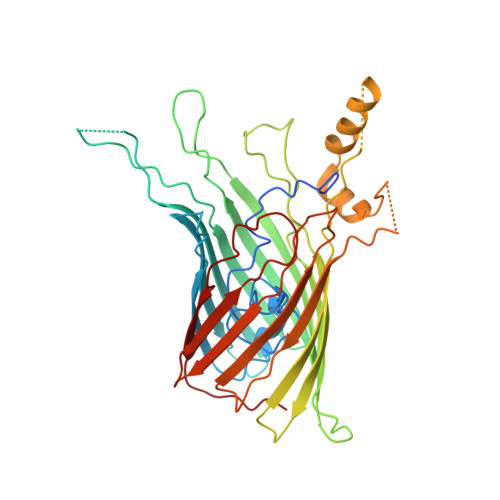
The type 9 secretion system (T9SS) is the protein export pathway of bacteria of the Gram-negative Fibrobacteres-Chlorobi-Bacteroidetes superphylum and is an essential determinant of pathogenicity in severe periodontal disease. The central element of the T9SS is a so-far uncharacterized protein-conducting translocon located in the bacterial outer membrane. Here, using cryo-electron microscopy, we provide structural evidence that the translocon is the T9SS protein SprA. SprA forms an extremely large (36-strand) single polypeptide transmembrane β-barrel. The barrel pore is capped on the extracellular end, but has a lateral opening to the external membrane surface. Structures of SprA bound to different components of the T9SS show that partner proteins control access to the lateral opening and to the periplasmic end of the pore. Our results identify a protein transporter with a distinctive architecture that uses an alternating access mechanism in which the two ends of the protein-conducting channel are open at different times.
Organizational Affiliation:
Department of Biochemistry, University of Oxford, Oxford, UK.