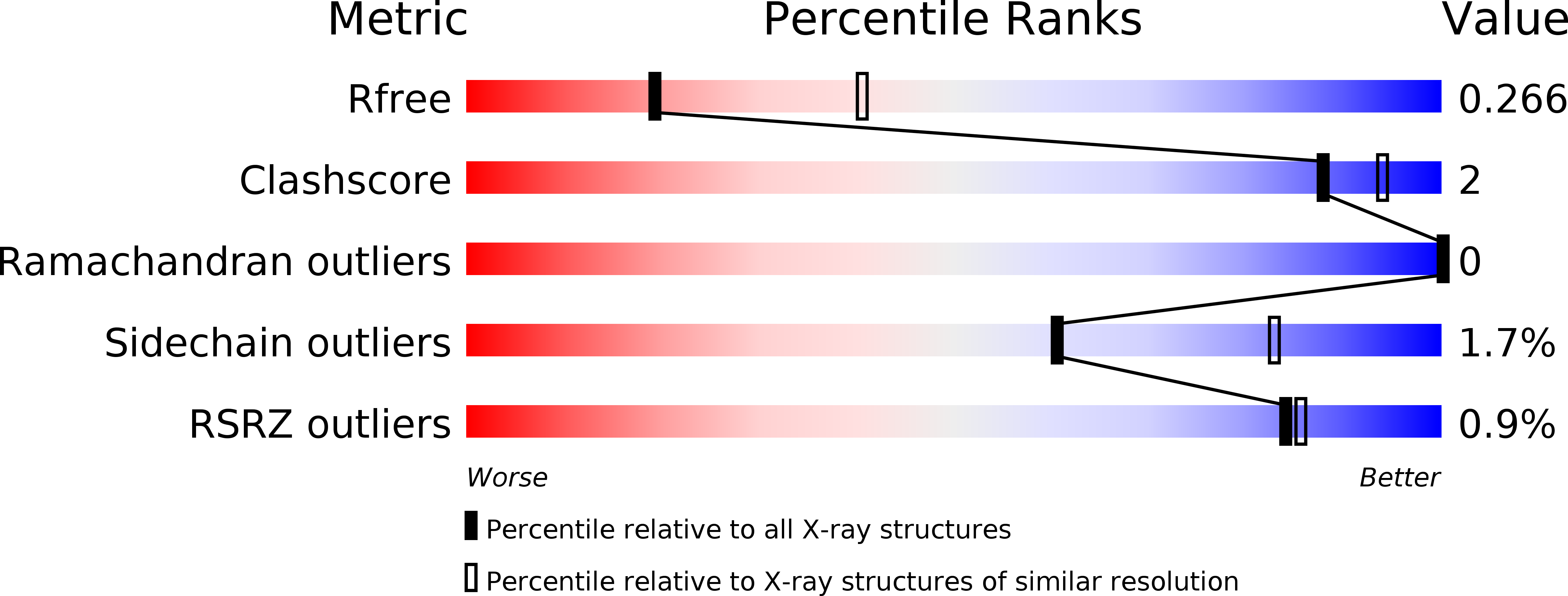
New Conformations of Acylation Adducts of Inhibitors of beta-Lactamase from Mycobacterium tuberculosis.
Tassoni, R., Blok, A., Pannu, N.S., Ubbink, M.(2019) Biochemistry 58: 997-1009
- PubMed: 30632739
- DOI: https://doi.org/10.1021/acs.biochem.8b01085
- Primary Citation of Related Structures:
6H27, 6H28, 6H2A, 6H2C, 6H2G, 6H2H, 6H2I, 6H2K - PubMed Abstract:
Mycobacterium tuberculosis (Mtb), the main causative agent of tuberculosis (TB), is naturally resistant to β-lactam antibiotics due to the production of the extended spectrum β-lactamase BlaC. β-Lactam/β-lactamase inhibitor combination therapies can circumvent the BlaC-mediated resistance of Mtb and are promising treatment options against TB. However, still little is known of the exact mechanism of BlaC inhibition by the β-lactamase inhibitors currently approved for clinical use, clavulanic acid, sulbactam, tazobactam, and avibactam. Here, we present the X-ray diffraction crystal structures of the acyl-enzyme adducts of wild-type BlaC with the four inhibitors. The +70 Da adduct derived from clavulanate and the trans-enamine acylation adducts of sulbactam and tazobactam are reported. BlaC in complex with avibactam revealed two inhibitor conformations. Preacylation binding could not be observed because inhibitor binding was not detected in BlaC variants carrying a substitution of the active site serine 70 to either alanine or cysteine, by crystallography, ITC or NMR. These results suggest that the catalytic serine 70 is necessary not only for enzyme acylation but also for increasing BlaC affinity for inhibitors in the preacylation state. The structure of BlaC with the serine to cysteine mutation showed a covalent linkage of the cysteine 70 Sγ atom to the nearby amino group of lysine 73. The differences of adduct conformations between BlaC and other β-lactamases are discussed.
Organizational Affiliation:
Leiden Institute of Chemistry , Leiden University , Einsteinweg 55 , Leiden 2333CC , The Netherlands.