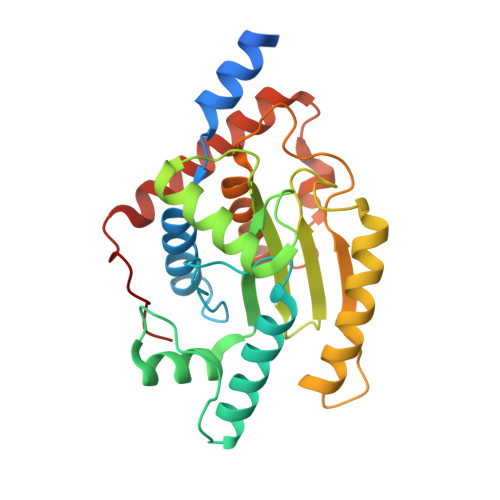
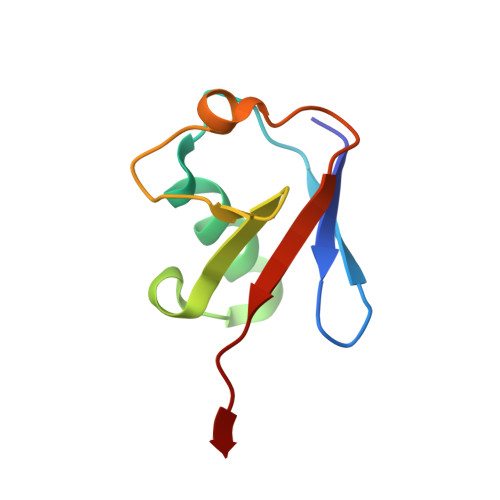
A Chlamydia effector combining deubiquitination and acetylation activities induces Golgi fragmentation.
Pruneda, J.N., Bastidas, R.J., Bertsoulaki, E., Swatek, K.N., Santhanam, B., Clague, M.J., Valdivia, R.H., Urbe, S., Komander, D.(2018) Nat Microbiol 3: 1377-1384
- PubMed: 30397340
- DOI: https://doi.org/10.1038/s41564-018-0271-y
- Primary Citation of Related Structures:
6GZS, 6GZT, 6GZU - PubMed Abstract:
Pathogenic bacteria are armed with potent effector proteins that subvert host signalling processes during infection 1 . The activities of bacterial effectors and their associated roles within the host cell are often poorly understood, particularly for Chlamydia trachomatis 2 , a World Health Organization designated neglected disease pathogen. We identify and explain remarkable dual Lys63-deubiquitinase (DUB) and Lys-acetyltransferase activities in the Chlamydia effector ChlaDUB1. Crystal structures capturing intermediate stages of each reaction reveal how the same catalytic centre of ChlaDUB1 can facilitate such distinct processes, and enable the generation of mutations that uncouple the two activities. Targeted Chlamydia mutant strains allow us to link the DUB activity of ChlaDUB1 and the related, dedicated DUB ChlaDUB2 to fragmentation of the host Golgi apparatus, a key process in Chlamydia infection for which effectors have remained elusive. Our work illustrates the incredible versatility of bacterial effector proteins, and provides important insights towards understanding Chlamydia pathogenesis.
Organizational Affiliation:
Division of Protein and Nucleic Acid Chemistry, MRC Laboratory of Molecular Biology, Cambridge, UK.