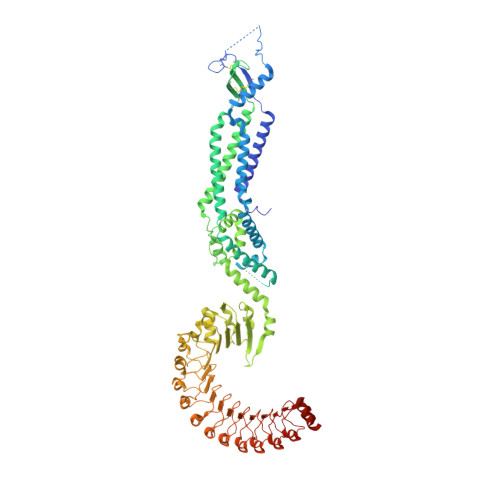
Structure of a volume-regulated anion channel of the LRRC8 family.
Deneka, D., Sawicka, M., Lam, A.K.M., Paulino, C., Dutzler, R.(2018) Nature 558: 254-259
- PubMed: 29769723
- DOI: https://doi.org/10.1038/s41586-018-0134-y
- Primary Citation of Related Structures:
6FNW, 6G8Z, 6G9L, 6G9O - PubMed Abstract:
Volume-regulated anion channels are activated in response to hypotonic stress. These channels are composed of closely related paralogues of the leucine-rich repeat-containing protein 8 (LRRC8) family that co-assemble to form hexameric complexes. Here, using cryo-electron microscopy and X-ray crystallography, we determine the structure of a homomeric channel of the obligatory subunit LRRC8A. This protein conducts ions and has properties in common with endogenous heteromeric channels. Its modular structure consists of a transmembrane pore domain followed by a cytoplasmic leucine-rich repeat domain. The transmembrane domain, which is structurally related to connexin proteins, is wide towards the cytoplasm but constricted on the outside by a structural unit that acts as a selectivity filter. An excess of basic residues in the filter and throughout the pore attracts anions by electrostatic interaction. Our work reveals the previously unknown architecture of volume-regulated anion channels and their mechanism of selective anion conduction.
Organizational Affiliation:
Department of Biochemistry, University of Zurich, Zurich, Switzerland.