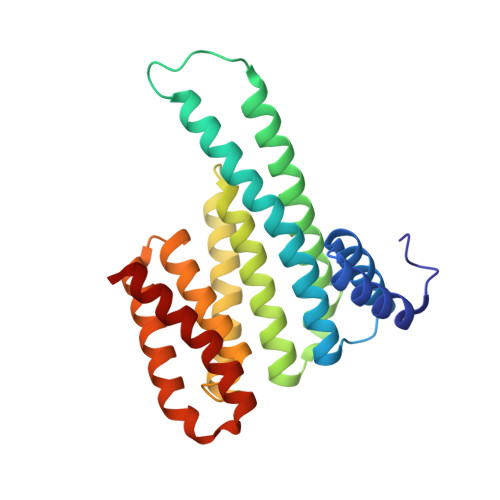
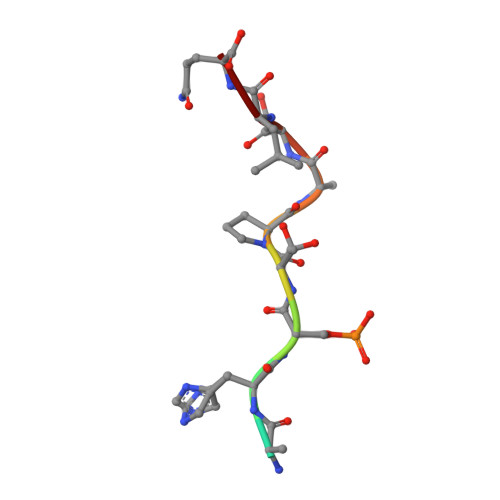
A study on the effect of synthetic alpha-to-beta3-amino acid mutations on the binding of phosphopeptides to 14-3-3 proteins.
Andrei, S.A., Thijssen, V., Brunsveld, L., Ottmann, C., Milroy, L.G.(2019) Chem Commun (Camb) 55: 14809-14812
- PubMed: 31763628
- DOI: https://doi.org/10.1039/c9cc07982c
- Primary Citation of Related Structures:
6G6X, 6G8I, 6G8J, 6G8K, 6G8L, 6G8P, 6G8Q - PubMed Abstract:
Here we describe the synthesis of a series of α,β-phosphopeptides, based on the phosphoepitope site on YAP1 (yes-associated protein 1), and the biochemical, biophysical and structural characterization of their binding to 14-3-3 proteins. The impact of systematic mono- and di-substitution of α → β3 amino acid residues around the phosphoserine residue are discussed. Our results confirm the important role played by the +2 proline residue in the thermodynamics and structure of the phosphoepitope/14-3-3 interaction.
Organizational Affiliation:
Laboratory of Chemical Biology, Department of Biomedical Engineering and Institute for Complex Molecular Systems (ICMS), Eindhoven University of Technology, 5600 MB Eindhoven, The Netherlands. l.brunsveld@tue.nl c.ottmann@tue.nl l.milroy@cantab.net.