High-resolution glycosylation site-engineering method identifies MICA epitope critical for shedding inhibition activity of anti-MICA antibodies.
Lombana, T.N., Matsumoto, M.L., Berkley, A.M., Toy, E., Cook, R., Gan, Y., Du, C., Schnier, P., Sandoval, W., Ye, Z., Schartner, J.M., Kim, J., Spiess, C.(2019) MAbs 11: 75-93
- PubMed: 30307368
- DOI: https://doi.org/10.1080/19420862.2018.1532767
- Primary Citation of Related Structures:
6DDM, 6DDR, 6DDV - PubMed Abstract:
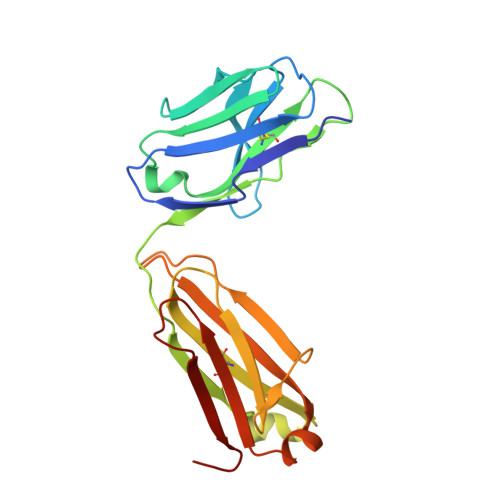
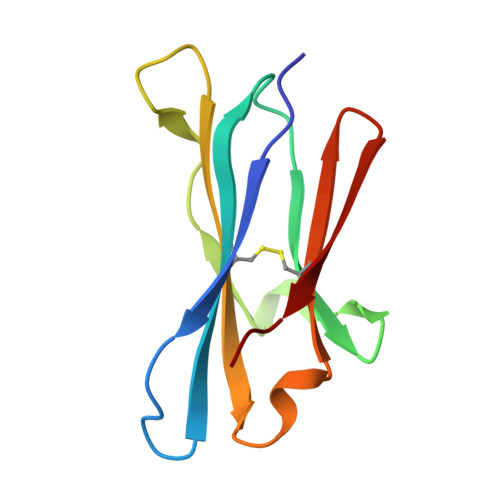
As an immune evasion strategy, MICA and MICB, the major histocompatibility complex class I homologs, are proteolytically cleaved from the surface of cancer cells leading to impairment of CD8 + T cell- and natural killer cell-mediated immune responses. Antibodies that inhibit MICA/B shedding from tumors have therapeutic potential, but the optimal epitopes are unknown. Therefore, we developed a high-resolution, high-throughput glycosylation-engineered epitope mapping (GEM) method, which utilizes site-specific insertion of N-linked glycans onto the antigen surface to mask local regions. We apply GEM to the discovery of epitopes important for shedding inhibition of MICA/B and validate the epitopes at the residue level by alanine scanning and X-ray crystallography (Protein Data Bank accession numbers 6DDM (1D5 Fab-MICA*008), 6DDR (13A9 Fab-MICA*008), 6DDV (6E1 Fab-MICA*008). Furthermore, we show that potent inhibition of MICA shedding can be achieved by antibodies that bind GEM epitopes adjacent to previously reported cleavage sites, and that these anti-MICA/B antibodies can prevent tumor growth in vivo.
Organizational Affiliation:
a Department of Antibody Engineering , Genentech Inc ., South San Francisco , USA.