Bromodomain-Selective BET Inhibitors Are Potent Antitumor Agents against MYC-Driven Pediatric Cancer.
Slavish, P.J., Chi, L., Yun, M.K., Tsurkan, L., Martinez, N.E., Jonchere, B., Chai, S.C., Connelly, M., Waddell, M.B., Das, S., Neale, G., Li, Z., Shadrick, W.R., Olsen, R.R., Freeman, K.W., Low, J.A., Price, J.E., Young, B.M., Bharatham, N., Boyd, V.A., Yang, J., Lee, R.E., Morfouace, M., Roussel, M.F., Chen, T., Savic, D., Guy, R.K., White, S.W., Shelat, A.A., Potter, P.M.(2020) Cancer Res 80: 3507-3518
- PubMed: 32651255
- DOI: https://doi.org/10.1158/0008-5472.CAN-19-3934
- Primary Citation of Related Structures:
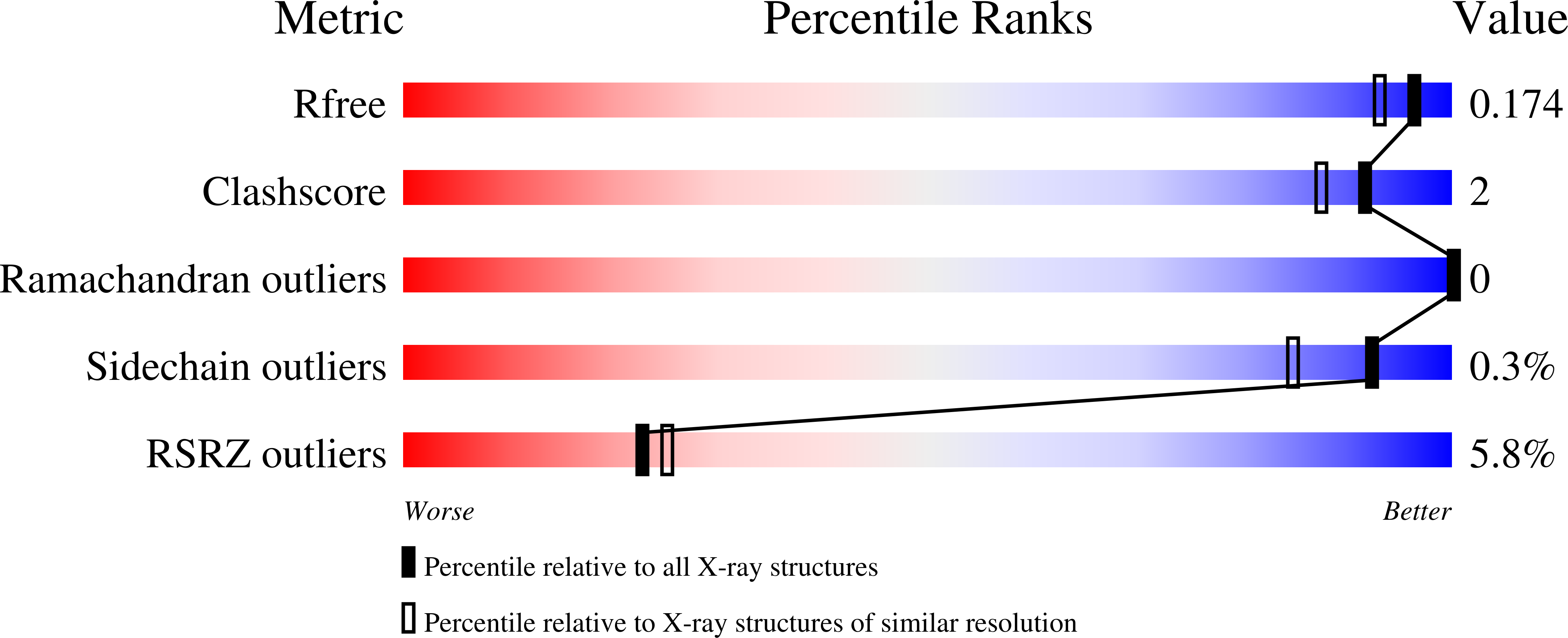
6DDI, 6DDJ - PubMed Abstract:
Inhibition of members of the bromodomain and extraterminal (BET) family of proteins has proven a valid strategy for cancer chemotherapy. All BET identified to date contain two bromodomains (BD; BD1 and BD2) that are necessary for recognition of acetylated lysine residues in the N-terminal regions of histones. Chemical matter that targets BET (BETi) also interact via these domains. Molecular and cellular data indicate that BD1 and BD2 have different biological roles depending upon their cellular context, with BD2 particularly associated with cancer. We have therefore pursued the development of BD2-selective molecules both as chemical probes and as potential leads for drug development. Here we report the structure-based generation of a novel series of tetrahydroquinoline analogs that exhibit >50-fold selectivity for BD2 versus BD1. This selective targeting resulted in engagement with BD-containing proteins in cells, resulting in modulation of MYC proteins and downstream targets. These compounds were potent cytotoxins toward numerous pediatric cancer cell lines and were minimally toxic to nontumorigenic cells. In addition, unlike the pan BETi (+)-JQ1, these BD2-selective inhibitors demonstrated no rebound expression effects. Finally, we report a pharmacokinetic-optimized, metabolically stable derivative that induced growth delay in a neuroblastoma xenograft model with minimal toxicity. We conclude that BD2-selective agents are valid candidates for antitumor drug design for pediatric malignancies driven by the MYC oncogene. SIGNIFICANCE: This study presents bromodomain-selective BET inhibitors that act as antitumor agents and demonstrates that these molecules have in vivo activity towards neuroblastoma, with essentially no toxicity.
Organizational Affiliation:
Department of Chemical Biology and Therapeutics, St. Jude Children's Research Hospital, Memphis, Tennessee.