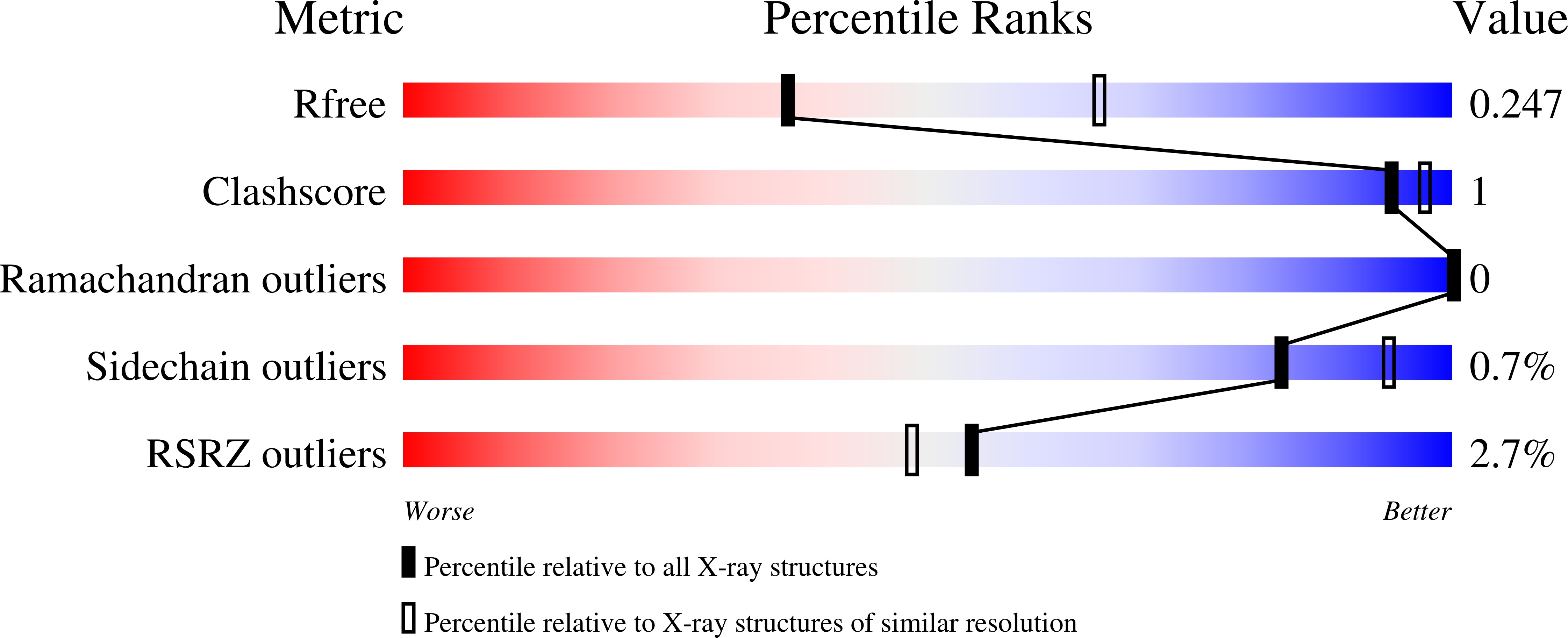
Assembly of human C-terminal binding protein (CtBP) into tetramers.
Bellesis, A.G., Jecrois, A.M., Hayes, J.A., Schiffer, C.A., Royer, W.E.(2018) J Biol Chem 293: 9101-9112
- PubMed: 29700119
- DOI: https://doi.org/10.1074/jbc.RA118.002514
- Primary Citation of Related Structures:
6CDF, 6CDR - PubMed Abstract:
C-terminal binding protein 1 (CtBP1) and CtBP2 are transcriptional coregulators that repress numerous cellular processes, such as apoptosis, by binding transcription factors and recruiting chromatin-remodeling enzymes to gene promoters. The NAD(H)-linked oligomerization of human CtBP is coupled to its co-transcriptional activity, which is implicated in cancer progression. However, the biologically relevant level of CtBP assembly has not been firmly established; nor has the stereochemical arrangement of the subunits above that of a dimer. Here, multi-angle light scattering (MALS) data established the NAD + - and NADH-dependent assembly of CtBP1 and CtBP2 into tetramers. An examination of subunit interactions within CtBP1 and CtBP2 crystal lattices revealed that both share a very similar tetrameric arrangement resulting from assembly of two dimeric pairs, with specific interactions probably being sensitive to NAD(H) binding. Creating a series of mutants of both CtBP1 and CtBP2, we tested the hypothesis that the crystallographically observed interdimer pairing stabilizes the solution tetramer. MALS data confirmed that these mutants disrupt both CtBP1 and CtBP2 tetramers, with the dimer generally remaining intact, providing the first stereochemical models for tetrameric assemblies of CtBP1 and CtBP2. The crystal structure of a subtle destabilizing mutant suggested that small structural perturbations of the hinge region linking the substrate- and NAD-binding domains are sufficient to weaken the CtBP1 tetramer. These results strongly suggest that the tetramer is important in CtBP function, and the series of CtBP mutants reported here can be used to investigate the physiological role of the tetramer.
Organizational Affiliation:
From the Department of Biochemistry and Molecular Pharmacology, University of Massachusetts Medical School, Worcester, Massachusetts 01605 and.