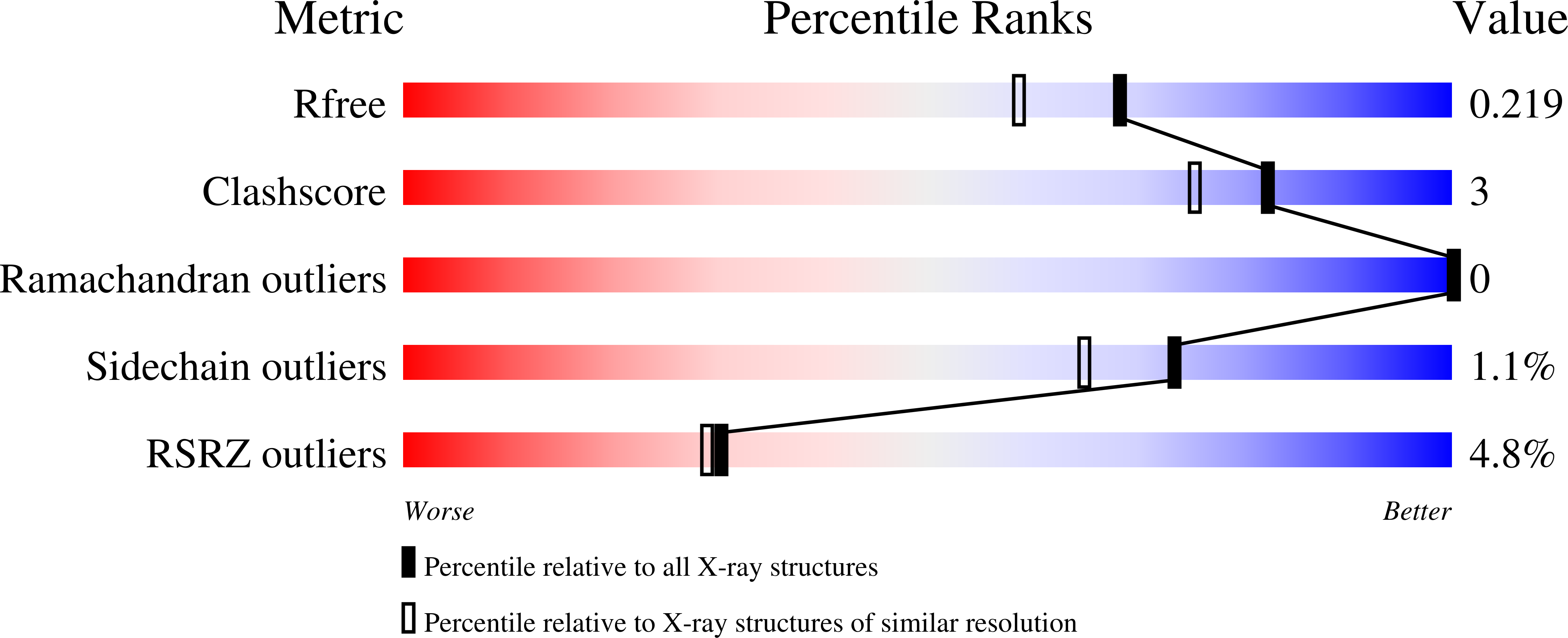
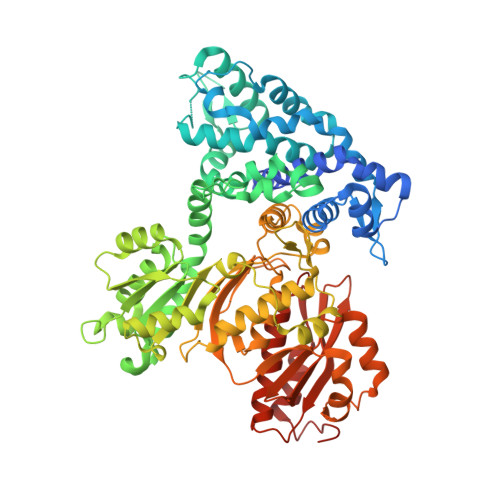
A Mononuclear Iron-Dependent Methyltransferase Catalyzes Initial Steps in Assembly of the Apratoxin A Polyketide Starter Unit.
Skiba, M.A., Sikkema, A.P., Moss, N.A., Tran, C.L., Sturgis, R.M., Gerwick, L., Gerwick, W.H., Sherman, D.H., Smith, J.L.(2017) ACS Chem Biol 12: 3039-3048
- PubMed: 29096064
- DOI: https://doi.org/10.1021/acschembio.7b00746
- Primary Citation of Related Structures:
6B39, 6B3A, 6B3B - PubMed Abstract:
Natural product biosynthetic pathways contain a plethora of enzymatic tools to carry out difficult biosynthetic transformations. Here, we discover an unusual mononuclear iron-dependent methyltransferase that acts in the initiation steps of apratoxin A biosynthesis (AprA MT1). Fe 3+ -replete AprA MT1 catalyzes one or two methyl transfer reactions on the substrate malonyl-ACP (acyl carrier protein), whereas Co 2+ , Fe 2+ , Mn 2+ , and Ni 2+ support only a single methyl transfer. MT1 homologues exist within the "GNAT" (GCN5-related N-acetyltransferase) loading modules of several modular biosynthetic pathways with propionyl, isobutyryl, or pivaloyl starter units. GNAT domains are thought to catalyze decarboxylation of malonyl-CoA and acetyl transfer to a carrier protein. In AprA, the GNAT domain lacks both decarboxylation and acyl transfer activity. A crystal structure of the AprA MT1-GNAT di-domain with bound Mn 2+ , malonate, and the methyl donor S-adenosylmethionine (SAM) reveals that the malonyl substrate is a bidentate metal ligand, indicating that the metal acts as a Lewis acid to promote methylation of the malonyl α-carbon. The GNAT domain is truncated relative to functional homologues. These results afford an expanded understanding of MT1-GNAT structure and activity and permit the functional annotation of homologous GNAT loading modules both with and without methyltransferases, additionally revealing their rapid evolutionary adaptation in different biosynthetic contexts.
Organizational Affiliation:
Life Sciences Institute, University of Michigan , Ann Arbor, Michigan 48109, United States.