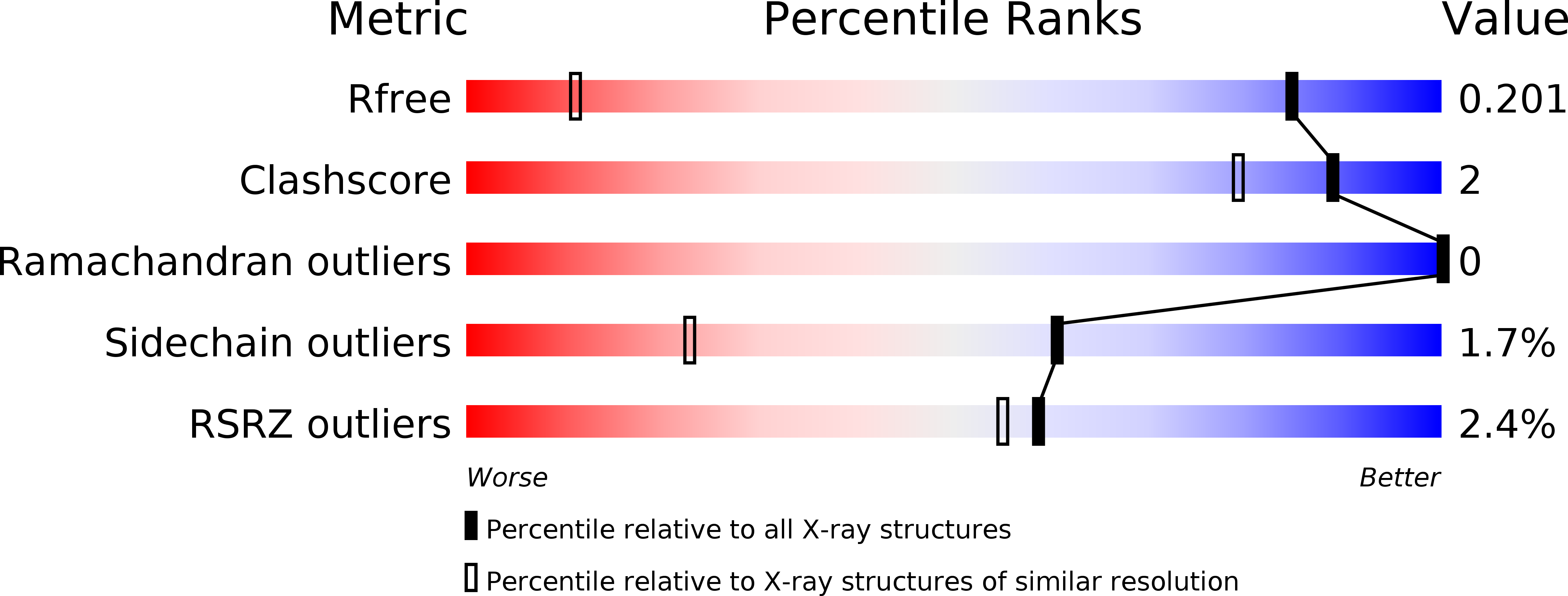
PI by NMR: Probing CH-pi Interactions in Protein-Ligand Complexes by NMR Spectroscopy.
Platzer, G., Mayer, M., Beier, A., Bruschweiler, S., Fuchs, J.E., Engelhardt, H., Geist, L., Bader, G., Schorghuber, J., Lichtenecker, R., Wolkerstorfer, B., Kessler, D., McConnell, D.B., Konrat, R.(2020) Angew Chem Int Ed Engl 59: 14861-14868
- PubMed: 32421895
- DOI: https://doi.org/10.1002/anie.202003732
- Primary Citation of Related Structures:
6XUZ, 6XV3, 6XV7, 6XVC - PubMed Abstract:
While CH-π interactions with target proteins are crucial determinants for the affinity of arguably every drug molecule, no method exists to directly measure the strength of individual CH-π interactions in drug-protein complexes. Herein, we present a fast and reliable methodology called PI (π interactions) by NMR, which can differentiate the strength of protein-ligand CH-π interactions in solution. By combining selective amino-acid side-chain labeling with 1 H- 13 C NMR, we are able to identify specific protein protons of side-chains engaged in CH-π interactions with aromatic ring systems of a ligand, based solely on 1 H chemical-shift values of the interacting protein aromatic ring protons. The information encoded in the chemical shifts induced by such interactions serves as a proxy for the strength of each individual CH-π interaction. PI by NMR changes the paradigm by which chemists can optimize the potency of drug candidates: direct determination of individual π interactions rather than averaged measures of all interactions.
Organizational Affiliation:
Christian Doppler Laboratory for High-Content Structural Biology and Biotechnology, Department of Structural and Computational Biology, Max Perutz Labs, University of Vienna, Campus Vienna Biocenter 5, 1030, Vienna, Austria.