Functional and Structural Analyses of the Split-Dehydratase Domain in the Biosynthesis of Macrolactam Polyketide Cremimycin.
Kawasaki, D., Miyanaga, A., Chisuga, T., Kudo, F., Eguchi, T.(2019) Biochemistry 58: 4799-4803
- PubMed: 31721563
- DOI: https://doi.org/10.1021/acs.biochem.9b00897
- Primary Citation of Related Structures:
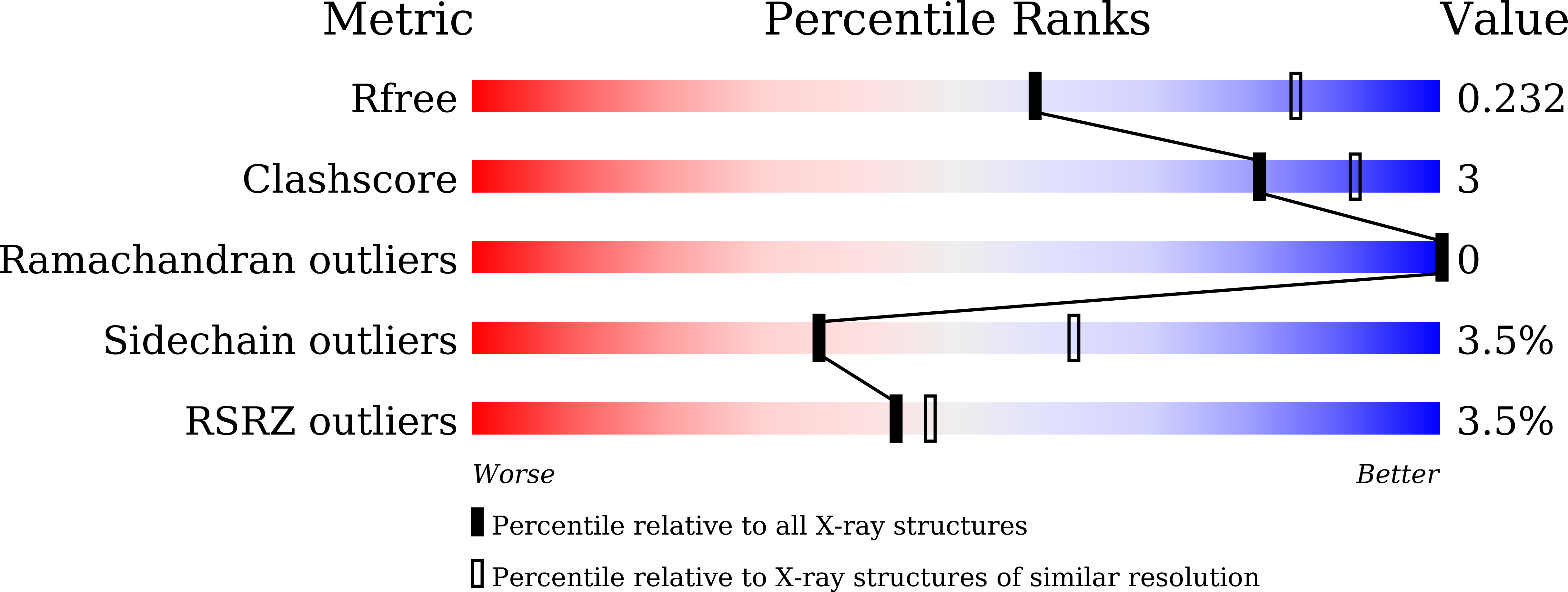
6K97 - PubMed Abstract:
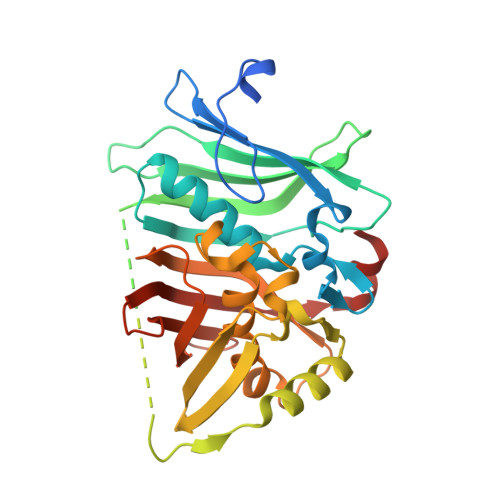
In the biosynthesis of the macrolactam antibiotic cremimycin, the 3-aminononanoic acid starter unit is formed via a non-2-enoyl acyl carrier protein thioester intermediate, which is presumed to be constructed by cis -acyltransferase (AT) polyketide synthases (PKSs) CmiP2, CmiP3, and CmiP4. While canonical cis -AT PKS modules are comprised of a single polypeptide, the PKS module formed by CmiP2 and CmiP3 is split within the dehydratase (DH) domain. Here, we report the enzymatic function and the structural features of this split-DH domain. In vitro analysis showed that the split-DH domain catalyzes the dehydration reaction of ( R )-3-hydroxynonanoyl N -acetylcysteamine thioester (SNAC) to form ( E )-non-2-enoyl-SNAC, suggesting that the split-DH domain is catalytically active in cremimycin biosynthesis. In addition, structural analysis revealed that the CmiP2 and CmiP3 subunits of the split-DH domain form a tightly associated heterodimer through several hydrogen bonding and hydrophobic interactions, which are similar to those of canonical DH domains of other cis -AT PKSs. These results indicate that the split-DH domain has the same function and structure as common cis -AT PKS DH domains.
Organizational Affiliation:
Department of Chemistry , Tokyo Institute of Technology , 2-12-1 O-okayama , Meguro-ku, Tokyo 152-8551 , Japan.