Molecular basis of egg coat cross-linking sheds light on ZP1-associated female infertility.
Nishimura, K., Dioguardi, E., Nishio, S., Villa, A., Han, L., Matsuda, T., Jovine, L.(2019) Nat Commun 10: 3086-3086
- PubMed: 31300655
- DOI: https://doi.org/10.1038/s41467-019-10931-5
- Primary Citation of Related Structures:
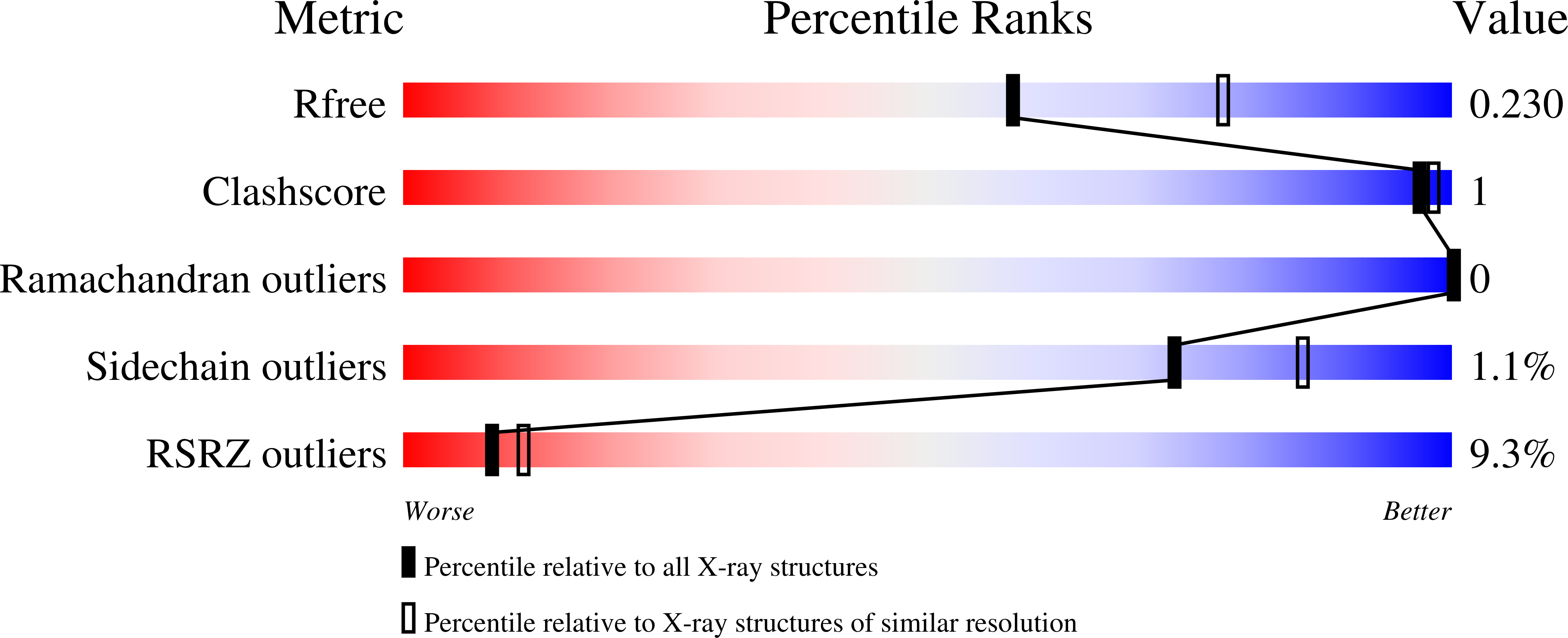
6GF6, 6GF7, 6GF8 - PubMed Abstract:
Mammalian fertilisation begins when sperm interacts with the egg zona pellucida (ZP), whose ZP1 subunit is important for fertility by covalently cross-linking ZP filaments into a three-dimensional matrix. Like ZP4, a structurally-related component absent in the mouse, ZP1 is predicted to contain an N-terminal ZP-N domain of unknown function. Here we report a characterisation of ZP1 proteins carrying mutations from infertile patients, which suggests that, in human, filament cross-linking by ZP1 is crucial to form a stable ZP. We map the function of ZP1 to its ZP-N1 domain and determine crystal structures of ZP-N1 homodimers from a chicken homolog of ZP1. These reveal that ZP filament cross-linking is highly plastic and can be modulated by ZP1 fucosylation and, potentially, zinc sparks. Moreover, we show that ZP4 ZP-N1 forms non-covalent homodimers in chicken but not in human. Together, these data identify human ZP1 cross-links as a promising target for non-hormonal contraception.
Organizational Affiliation:
Department of Biosciences and Nutrition and Center for Innovative Medicine, Karolinska Institutet, Huddinge, SE-141 83, Sweden.