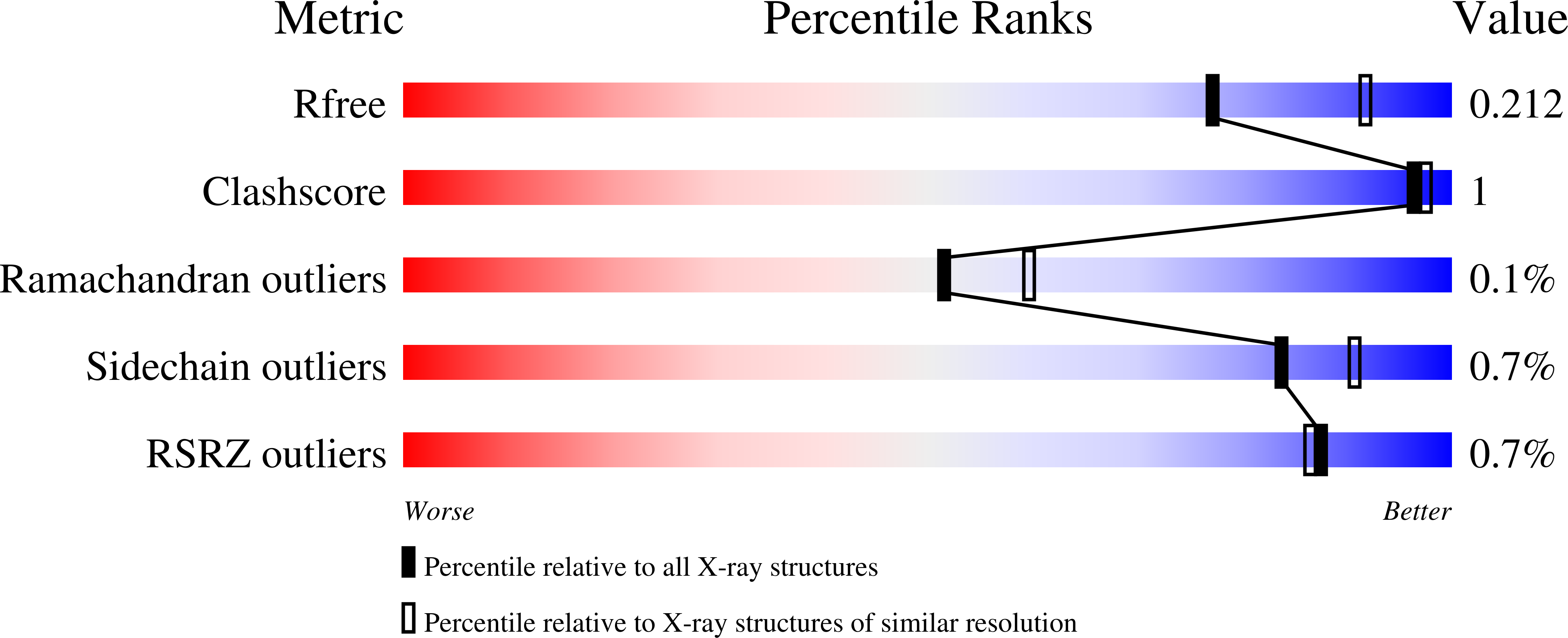
Novel Scaffolds for Dual Specificity Tyrosine-Phosphorylation-Regulated Kinase (DYRK1A) Inhibitors.
Czarna, A., Wang, J., Zelencova, D., Liu, Y., Deng, X., Choi, H.G., Zhang, T., Zhou, W., Chang, J.W., Kildalsen, H., Seternes, O.M., Gray, N.S., Engh, R.A., Rothweiler, U.(2018) J Med Chem 61: 7560-7572
- PubMed: 30095246
- DOI: https://doi.org/10.1021/acs.jmedchem.7b01847
- Primary Citation of Related Structures:
6EIF, 6EIJ, 6EIL, 6EIP, 6EIQ, 6EIR, 6EIS, 6EIV, 6EJ4 - PubMed Abstract:
DYRK1A is one of five members of the dual-specificity tyrosine (Y) phosphorylation-regulated kinase (DYRK) family. The DYRK1A gene is located in the Down syndrome critical region and regulates cellular processes related to proliferation and differentiation of neuronal progenitor cells during early development. This has focused research on its role in neuronal degenerative diseases, including Alzheimer's and Down syndrome. Recent studies have also shown a possible role of DYRK1A in diabetes. Here we report a variety of scaffolds not generally known for DYRK1A inhibition, demonstrating their effects in in vitro assays and also in cell cultures. These inhibitors effectively block the tau phosphorylation that is a hallmark of Alzheimer's disease. The crystal structures of these inhibitors support the design of optimized and novel therapeutics.
Organizational Affiliation:
Department of Pharmacy, Faculty of Health Sciences , UiT The Arctic University of Norway , N-9037 Tromsø , Norway.