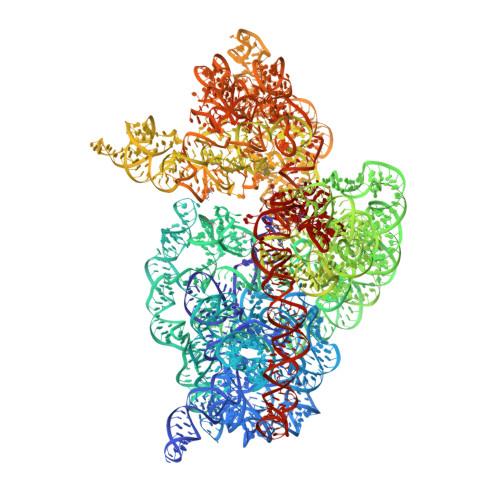
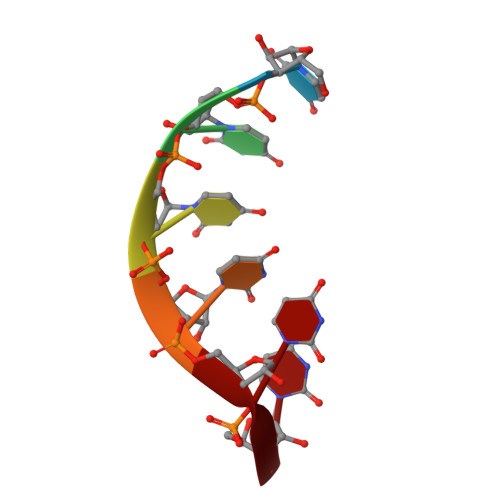
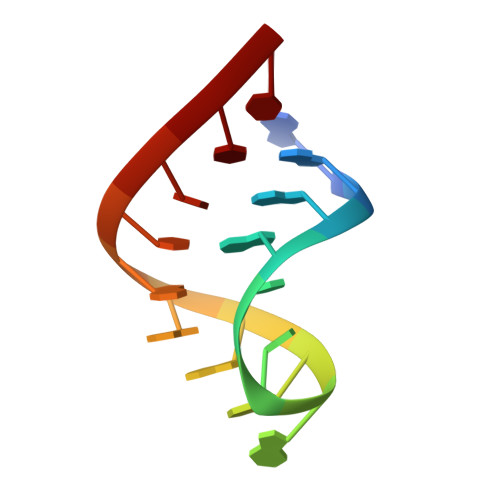
Aminoglycoside ribosome interactions reveal novel conformational states at ambient temperature.
O'Sullivan, M.E., Poitevin, F., Sierra, R.G., Gati, C., Dao, E.H., Rao, Y., Aksit, F., Ciftci, H., Corsepius, N., Greenhouse, R., Hayes, B., Hunter, M.S., Liang, M., McGurk, A., Mbgam, P., Obrinsky, T., Pardo-Avila, F., Seaberg, M.H., Cheng, A.G., Ricci, A.J., DeMirci, H.(2018) Nucleic Acids Res 46: 9793-9804
- PubMed: 30113694
- DOI: https://doi.org/10.1093/nar/gky693
- Primary Citation of Related Structures:
6CAR, 6CAS - PubMed Abstract:
The bacterial 30S ribosomal subunit is a primary antibiotic target. Despite decades of discovery, the mechanisms by which antibiotic binding induces ribosomal dysfunction are not fully understood. Ambient temperature crystallographic techniques allow more biologically relevant investigation of how local antibiotic binding site interactions trigger global subunit rearrangements that perturb protein synthesis. Here, the structural effects of 2-deoxystreptamine (paromomycin and sisomicin), a novel sisomicin derivative, N1-methyl sulfonyl sisomicin (N1MS) and the non-deoxystreptamine (streptomycin) aminoglycosides on the ribosome at ambient and cryogenic temperatures were examined. Comparative studies led to three main observations. First, individual aminoglycoside-ribosome interactions in the decoding center were similar for cryogenic versus ambient temperature structures. Second, analysis of a highly conserved GGAA tetraloop of h45 revealed aminoglycoside-specific conformational changes, which are affected by temperature only for N1MS. We report the h44-h45 interface in varying states, i.e. engaged, disengaged and in equilibrium. Third, we observe aminoglycoside-induced effects on 30S domain closure, including a novel intermediary closure state, which is also sensitive to temperature. Analysis of three ambient and five cryogenic crystallography datasets reveal a correlation between h44-h45 engagement and domain closure. These observations illustrate the role of ambient temperature crystallography in identifying dynamic mechanisms of ribosomal dysfunction induced by local drug-binding site interactions. Together, these data identify tertiary ribosomal structural changes induced by aminoglycoside binding that provides functional insight and targets for drug design.
Organizational Affiliation:
Department of Otolaryngology-Head and Neck Surgery, Stanford University School of Medicine, Palo Alto, CA, USA, 94305.