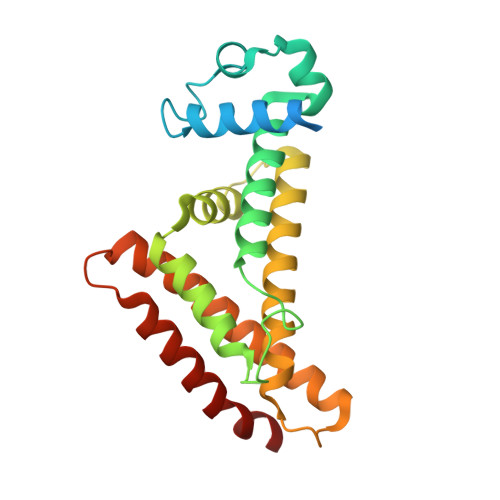
The crystal structure of AcrR from Mycobacterium tuberculosis reveals a one-component transcriptional regulation mechanism.
Kang, S.M., Kim, D.H., Jin, C., Ahn, H.C., Lee, B.J.(2019) FEBS Open Bio 9: 1713-1725
- PubMed: 31369208
- DOI: https://doi.org/10.1002/2211-5463.12710
- Primary Citation of Related Structures:
6A4L, 6A4W - PubMed Abstract:
Transcriptional regulator proteins are closely involved in essential survival strategies in bacteria. AcrR is a one-component allosteric repressor of the genes associated with lipid transport and antibiotic resistance. When fatty acid ligands bind to the C-terminal ligand-binding cavity of AcrR, a conformational change in the N-terminal operator-binding region of AcrR is triggered, which releases the repressed DNA and initiates transcription. This paper focuses on the structural transition mechanism of AcrR of Mycobacterium tuberculosis upon DNA and ligand binding. AcrR loses its structural integrity upon ligand-mediated structural alteration and bends toward the promoter DNA in a more compact form, initiating a rotational motion. Our functional characterization of AcrR and description of the ligand- and DNA-recognition mechanism may facilitate the discovery of new therapies for tuberculosis.
Organizational Affiliation:
The Research Institute of Pharmaceutical Sciences, College of Pharmacy, Seoul National University, Seoul, Korea.