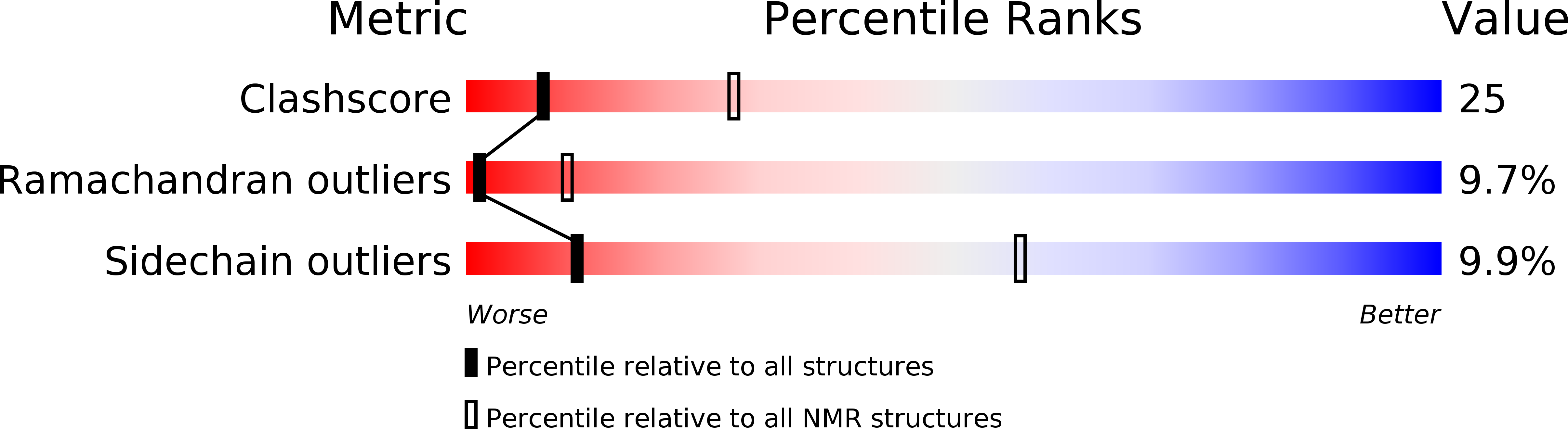Solution structure of the RNA recognition domain of METTL3-METTL14 N6-methyladenosine methyltransferase.
Huang, J., Dong, X., Gong, Z., Qin, L.Y., Yang, S., Zhu, Y.L., Wang, X., Zhang, D., Zou, T., Yin, P., Tang, C.(2019) Protein Cell 10: 272-284
- PubMed: 29542011
- DOI: https://doi.org/10.1007/s13238-018-0518-7
- Primary Citation of Related Structures:
5YZ9 - PubMed Abstract:
N 6 -methyladenosine (m 6 A), a ubiquitous RNA modification, is installed by METTL3-METTL14 complex. The structure of the heterodimeric complex between the methyltransferase domains (MTDs) of METTL3 and METTL14 has been previously determined. However, the MTDs alone possess no enzymatic activity. Here we present the solution structure for the zinc finger domain (ZFD) of METTL3, the inclusion of which fulfills the methyltransferase activity of METTL3-METTL14. We show that the ZFD specifically binds to an RNA containing 5'-GGACU-3' consensus sequence, but does not to one without. The ZFD thus serves as the target recognition domain, a structural feature previously shown for DNA methyltransferases, and cooperates with the MTDs of METTL3-METTL14 for catalysis. However, the interaction between the ZFD and the specific RNA is extremely weak, with the binding affinity at several hundred micromolar under physiological conditions. The ZFD contains two CCCH-type zinc fingers connected by an anti-parallel β-sheet. Mutational analysis and NMR titrations have mapped the functional interface to a contiguous surface. As a division of labor, the RNA-binding interface comprises basic residues from zinc finger 1 and hydrophobic residues from β-sheet and zinc finger 2. Further we show that the linker between the ZFD and MTD of METTL3 is flexible but partially folded, which may permit the cooperation between the two domains during catalysis. Together, the structural characterization of METTL3 ZFD paves the way to elucidate the atomic details of the entire process of RNA m 6 A modification.
Organizational Affiliation:
National Key Laboratory of Crop Genetic Improvement and National Center of Plant Gene Research, Huazhong Agricultural University, Wuhan, 430070, China.