Conformational Aspects in the Design of Inhibitors for Serine Hydroxymethyltransferase (SHMT): Biphenyl, Aryl Sulfonamide, and Aryl Sulfone Motifs
Schwertz, G., Frei, M.S., Witschel, M.C., Rottmann, M., Leartsakulpanich, U., Chitnumsub, P., Jaruwat, A., Ittarat, W., Schafer, A., Aponte, R.A., Trapp, N., Mark, K., Chaiyen, P., Diederich, F.(2017) Chemistry 23: 14345-14357
- PubMed: 28967982
- DOI: https://doi.org/10.1002/chem.201703244
- Primary Citation of Related Structures:
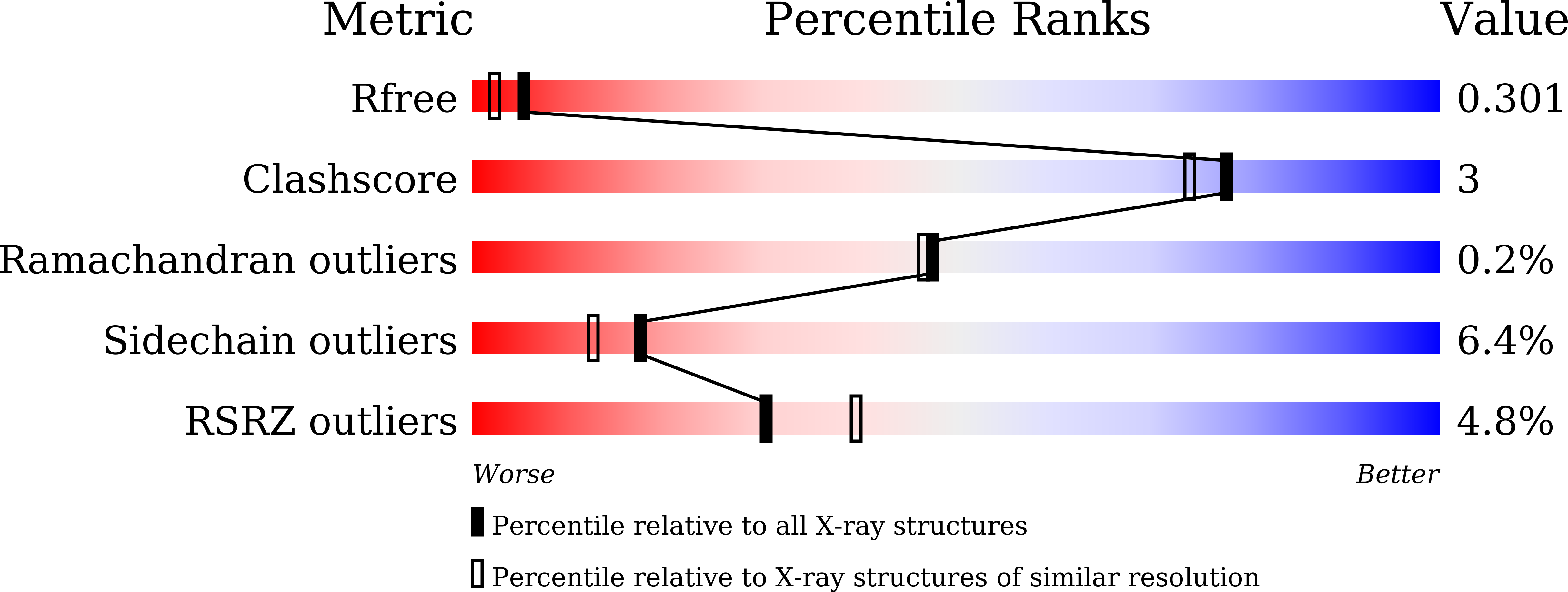
5XMP, 5XMQ, 5XMR, 5XMS, 5XMT, 5XMU, 5XMV - PubMed Abstract:
Malaria remains a major threat to mankind due to the perpetual emergence of resistance against marketed drugs. Twenty-one pyrazolopyran-based inhibitors bearing terminal biphenyl, aryl sulfonamide, or aryl sulfone motifs were synthesized and tested towards serine hydroxymethyltransferase (SHMT), a key enzyme of the folate cycle. The best ligands inhibited Plasmodium falciparum (Pf) and Arabidopsis thaliana (At) SHMT in target, as well as PfNF54 strains in cell-based assays in the low nanomolar range (18-56 nm). Seven co-crystal structures with P. vivax (Pv) SHMT were solved at 2.2-2.6 Å resolution. We observed an unprecedented influence of the torsion angle of ortho-substituted biphenyl moieties on cell-based efficacy. The peculiar lipophilic character of the sulfonyl moiety was highlighted in the complexes with aryl sulfonamide analogues, which bind in their preferred staggered orientation. The results are discussed within the context of conformational preferences in the ligands.
Organizational Affiliation:
Laboratorium für Organische Chemie, ETH Zurich, Vladimir-Prelog-Weg 3, 8093, Zurich, Switzerland.