Structural Insights into Modulation of Neurexin-Neuroligin Trans-synaptic Adhesion by MDGA1/Neuroligin-2 Complex
Kim, J.A., Kim, D., Won, S.Y., Han, K.A., Park, D., Cho, E., Yun, N., An, H.J., Um, J.W., Kim, E., Lee, J.O., Ko, J., Kim, H.M.(2017) Neuron 94: 1121-1131.e6
- PubMed: 28641111
- DOI: https://doi.org/10.1016/j.neuron.2017.05.034
- Primary Citation of Related Structures:
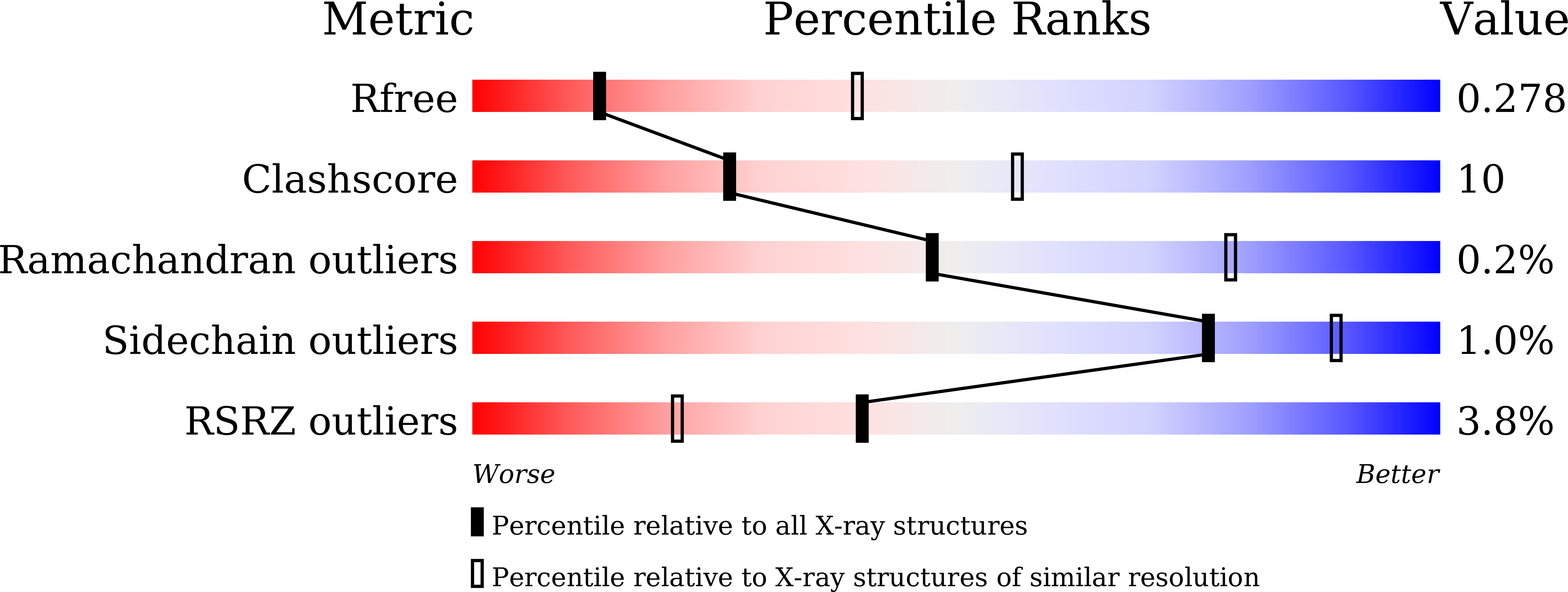
5XEQ - PubMed Abstract:
Membrane-associated mucin domain-containing glycosylphosphatidylinositol anchor proteins (MDGAs) bind directly to neuroligin-1 (NL1) and neuroligin-2 (NL2), thereby respectively regulating excitatory and inhibitory synapse development. However, the mechanisms by which MDGAs modulate NL activity to specify development of the two synapse types remain unclear. Here, we determined the crystal structures of human NL2/MDGA1 Ig1-3 complex, revealing their stable 2:2 arrangement with three interaction interfaces. Cell-based assays using structure-guided, site-directed MDGA1 mutants showed that all three contact patches were required for the MDGA's negative regulation of NL2-mediated synaptogenic activity. Furthermore, MDGA1 competed with neurexins for NL2 via its Ig1 domain. The binding affinities of both MDGA1 and MDGA2 for NL1 and NL2 were similar, consistent with the structural prediction of similar binding interfaces. However, MDGA1 selectively associated with NL2, but not NL1, in vivo. These findings collectively provide structural insights into the mechanism by which MDGAs negatively modulate synapse development governed by NLs/neurexins.
Organizational Affiliation:
Graduate School of Nanoscience and Technology, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Korea.