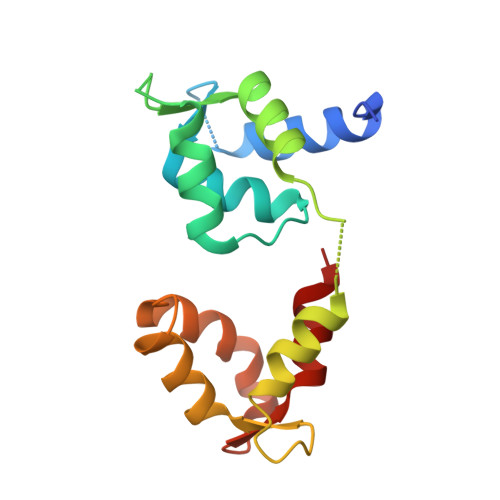
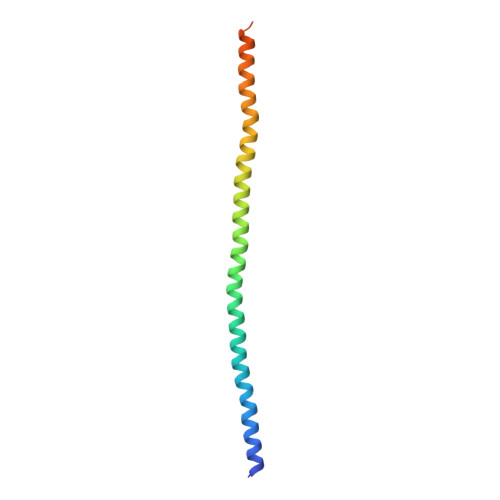
Ca(2+)-Induced Rigidity Change of the Myosin VIIa IQ Motif-Single alpha Helix Lever Arm Extension
Li, J., Chen, Y., Deng, Y., Unarta, I.C., Lu, Q., Huang, X., Zhang, M.(2017) Structure 25: 579-591.e4
- PubMed: 28262393
- DOI: https://doi.org/10.1016/j.str.2017.02.002
- Primary Citation of Related Structures:
5WST, 5WSU, 5WSV - PubMed Abstract:
Several unconventional myosins contain a highly charged single α helix (SAH) immediately following the calmodulin (CaM) binding IQ motifs, functioning to extend lever arms of these myosins. How such SAH is connected to the IQ motifs and whether the conformation of the IQ motifs-SAH segments are regulated by Ca 2+ fluctuations are not known. Here, we demonstrate by solving its crystal structure that the predicted SAH of myosin VIIa (Myo7a) forms a stable SAH. The structure of Myo7a IQ5-SAH segment in complex with apo-CaM reveals that the SAH sequence can extend the length of the Myo7a lever arm. Although Ca 2+ -CaM remains bound to IQ5-SAH, the Ca 2+ -induced CaM binding mode change softens the conformation of the IQ5-SAH junction, revealing a Ca 2+ -induced lever arm flexibility change for Myo7a. We further demonstrate that the last IQ motif of several other myosins also binds to both apo- and Ca 2+ -CaM, suggesting a common Ca 2+ -induced conformational regulation mechanism.
Organizational Affiliation:
Division of Life Science, State Key Laboratory of Molecular Neuroscience, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China. Electronic address: bcjli@connect.ust.hk.