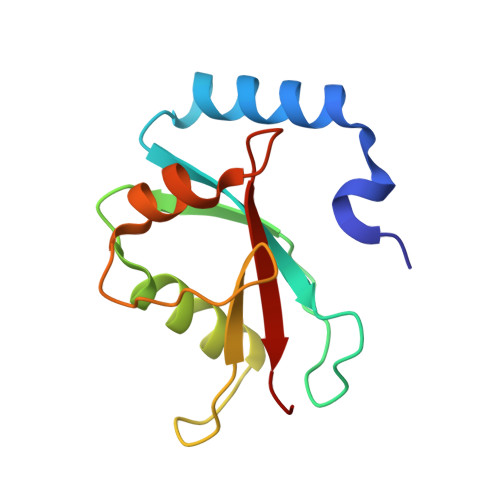
A helical LC3-interacting region mediates the interaction between the retroviral restriction factor Trim5 alpha and mammalian autophagy-related ATG8 proteins.
Keown, J.R., Black, M.M., Ferron, A., Yap, M., Barnett, M.J., Pearce, F.G., Stoye, J.P., Goldstone, D.C.(2018) J Biol Chem 293: 18378-18386
- PubMed: 30282803
- DOI: https://doi.org/10.1074/jbc.RA118.004202
- Primary Citation of Related Structures:
5W9A - PubMed Abstract:
The retroviral restriction factor tripartite motif-containing 5α (Trim5α) acts during the early postentry stages of the retroviral life cycle to block infection by a broad range of retroviruses, disrupting reverse transcription and integration. The mechanism of this restriction is poorly understood, but it has recently been suggested to involve recruitment of components of the autophagy machinery, including members of the mammalian autophagy-related 8 (ATG8) family involved in targeting proteins to the autophagosome. To better understand the molecular details of this interaction, here we utilized analytical ultracentrifugation to characterize the binding of six ATG8 isoforms and determined the crystal structure of the Trim5α Bbox coiled-coil region in complex with one member of the mammalian ATG8 proteins, autophagy-related protein LC3 B (LC3B). We found that Trim5α binds all mammalian ATG8s and that, unlike the typical LC3-interacting region (LIR) that binds to mammalian ATG8s through a β-strand motif comprising approximately six residues, LC3B binds to Trim5α via the α-helical coiled-coil region. The orientation of the structure demonstrated that LC3B could be accommodated within a Trim5α assembly that can bind the retroviral capsid. However, mutation of the binding interface does not affect retroviral restriction. Comparison of the typical linear β-strand LIR with our atypical helical LIR reveals a conservation of the presentation of residues that are required for the interaction with LC3B. This observation expands the range of LC3B-binding proteins to include helical binding motifs and demonstrates a link between Trim5α and components of the autophagosome.
Organizational Affiliation:
From the School of Biological Sciences, University of Auckland, Auckland 1010, New Zealand.