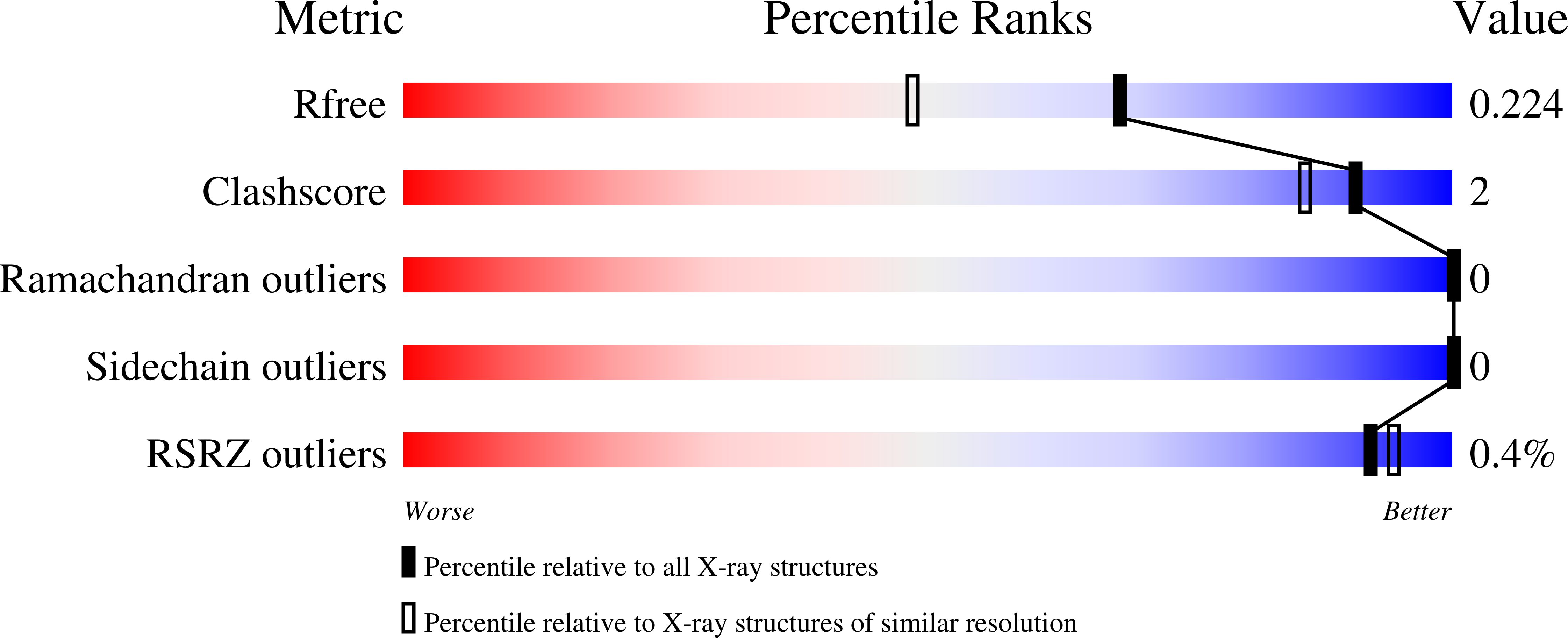
A competent catalytic active site is necessary for substrate induced dimer assembly in triosephosphate isomerase.
Jimenez-Sandoval, P., Vique-Sanchez, J.L., Hidalgo, M.L., Velazquez-Juarez, G., Diaz-Quezada, C., Arroyo-Navarro, L.F., Moran, G.M., Fattori, J., Jessica Diaz-Salazar, A., Rudino-Pinera, E., Sotelo-Mundo, R., Figueira, A.C.M., Lara-Gonzalez, S., Benitez-Cardoza, C.G., Brieba, L.G.(2017) Biochim Biophys Acta 1865: 1423-1432
- PubMed: 28803140
- DOI: https://doi.org/10.1016/j.bbapap.2017.07.014
- Primary Citation of Related Structures:
5VWN - PubMed Abstract:
The protozoan parasite Trichomonas vaginalis contains two nearly identical triosephosphate isomerases (TvTIMs) that dissociate into stable monomers and dimerize upon substrate binding. Herein, we compare the role of the "ball and socket" and loop 3 interactions in substrate assisted dimer assembly in both TvTIMs. We found that point mutants at the "ball" are only 39 and 29-fold less catalytically active than their corresponding wild-type counterparts, whereas Δloop 3 deletions are 1502 and 9400-fold less active. Point and deletion mutants dissociate into stable monomers. However, point mutants assemble as catalytic competent dimers upon binding of the transition state substrate analog PGH, whereas loop 3 deletions remain monomeric. A comparison between crystal structures of point and loop 3 deletion monomeric mutants illustrates that the catalytic residues in point mutants and wild-type TvTIMs are maintained in the same orientation, whereas the catalytic residues in deletion mutants show an increase in thermal mobility and present structural disorder that may hamper their catalytic role. The high enzymatic activity present in monomeric point mutants correlates with the formation of dimeric TvTIMs upon substrate binding. In contrast, the low activity and lack of dimer assembly in deletion mutants suggests a role of loop 3 in promoting the formation of the active site as well as dimer assembly. Our results suggest that in TvTIMs the active site is assembled during dimerization and that the integrity of loop 3 and ball and socket residues is crucial to stabilize the dimer.
Organizational Affiliation:
Laboratorio Nacional de Genómica para la Biodiversidad, Centro de Investigación y de Estudios Avanzados del IPN, Apartado Postal 629, Irapuato, CP 36821 Guanajuato, Mexico.