Crystal Structure of Beta-barrel-like Protein of Unknown Function
Kim, Y., Bigelow, L., Endres, M., Babnigg, G., Crosson, S., Joachimiak, A., Midwest Center for Structural Genomics (MCSG)To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Substrate-binding region of ABC-type glycine betaine transport system | 282 | Brucella abortus 2308 | Mutation(s): 0 Gene Names: BAB1_0226 | 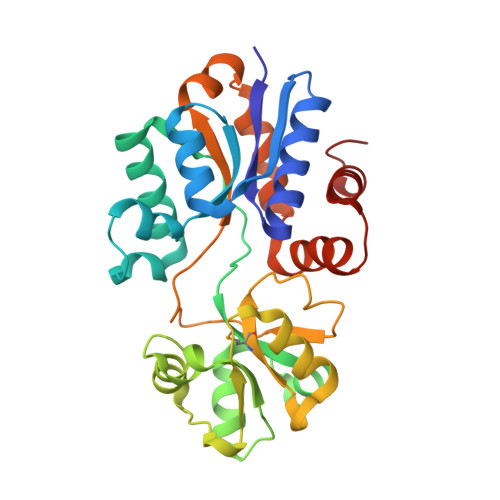 | |
UniProt | |||||
Find proteins for Q2YP76 (Brucella abortus (strain 2308)) Explore Q2YP76 Go to UniProtKB: Q2YP76 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q2YP76 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GOL Query on GOL | J [auth B], O [auth E] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| CA Query on CA | I [auth A] K [auth B] L [auth C] M [auth D] N [auth E] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B, C, D, E A, B, C, D, E, F, G, H | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 82.602 | α = 95.19 |
| b = 92.738 | β = 110.87 |
| c = 95.124 | γ = 111.35 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| HKL-3000 | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | -- |