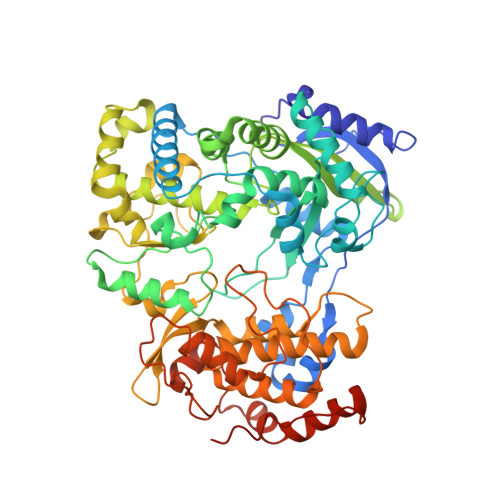
Structure and function of the Zika virus full-length NS5 protein.
Zhao, B., Yi, G., Du, F., Chuang, Y.C., Vaughan, R.C., Sankaran, B., Kao, C.C., Li, P.(2017) Nat Commun 8: 14762-14762
- PubMed: 28345656
- DOI: https://doi.org/10.1038/ncomms14762
- Primary Citation of Related Structures:
5U0B, 5U0C - PubMed Abstract:
The recent outbreak of Zika virus (ZIKV) has infected over 1 million people in over 30 countries. ZIKV replicates its RNA genome using virally encoded replication proteins. Nonstructural protein 5 (NS5) contains a methyltransferase for RNA capping and a polymerase for viral RNA synthesis. Here we report the crystal structures of full-length NS5 and its polymerase domain at 3.0 Å resolution. The NS5 structure has striking similarities to the NS5 protein of the related Japanese encephalitis virus. The methyltransferase contains in-line pockets for substrate binding and the active site. Key residues in the polymerase are located in similar positions to those of the initiation complex for the hepatitis C virus polymerase. The polymerase conformation is affected by the methyltransferase, which enables a more efficiently elongation of RNA synthesis in vitro. Overall, our results will contribute to future studies on ZIKV infection and the development of inhibitors of ZIKV replication.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Texas A&M University, College Station, Texas 77843, USA.