Molecular basis for the behavioral effects of the odorant degrading enzyme Esterase 6 in Drosophila.
Younus, F., Fraser, N.J., Coppin, C.W., Liu, J.W., Correy, G.J., Chertemps, T., Pandey, G., Maibeche, M., Jackson, C.J., Oakeshott, J.G.(2017) Sci Rep 7: 46188-46188
- PubMed: 28393888
- DOI: https://doi.org/10.1038/srep46188
- Primary Citation of Related Structures:
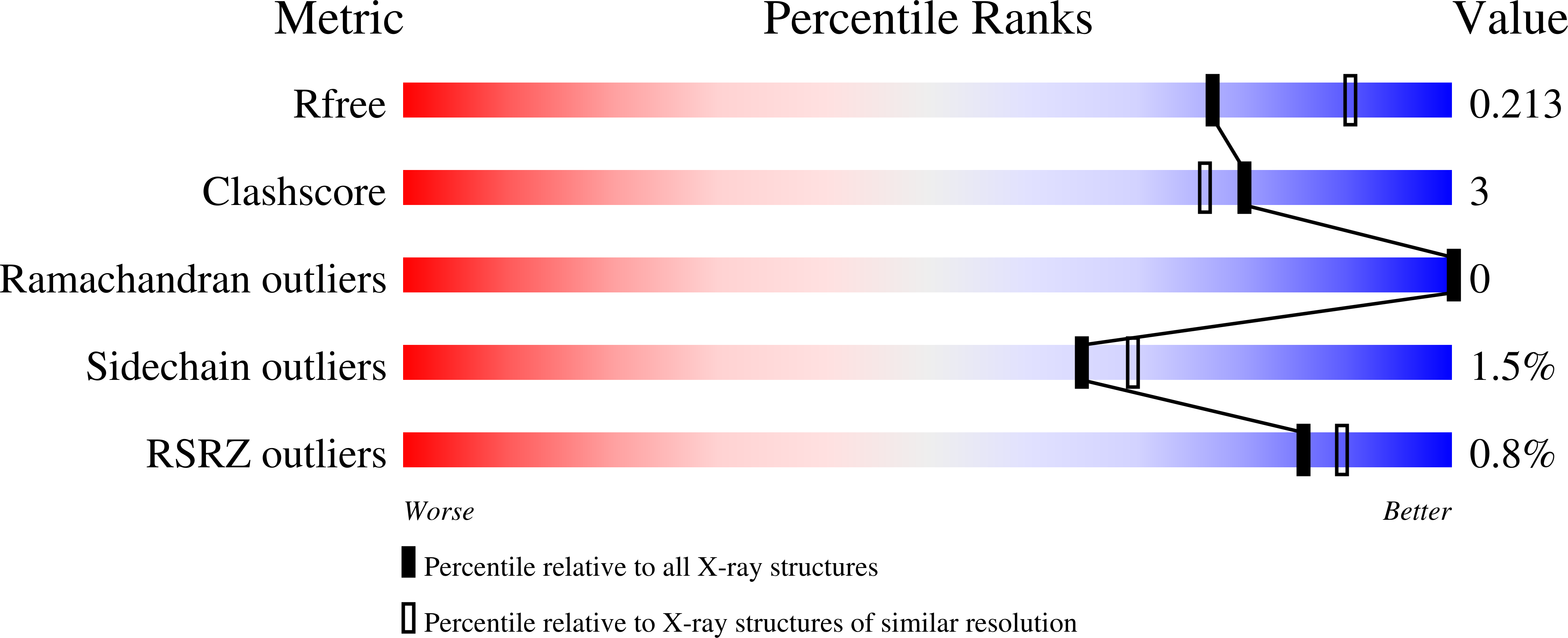
5THM - PubMed Abstract:
Previous electrophysiological and behavioural studies implicate esterase 6 in the processing of the pheromone cis-vaccenyl acetate and various food odorants that affect aggregation and reproductive behaviours. Here we show esterase 6 has relatively high activity against many of the short-mid chain food esters, but negligible activity against cis-vaccenyl acetate. The crystal structure of esterase 6 confirms its substrate-binding site can accommodate many short-mid chain food esters but not cis-vaccenyl acetate. Immunohistochemical assays show esterase 6 is expressed in non-neuronal cells in the third antennal segment that could be accessory or epidermal cells surrounding numerous olfactory sensilla, including basiconics involved in food odorant detection. Esterase 6 is also produced in trichoid sensilla, but not in the same cell types as the cis-vaccenyl acetate binding protein LUSH. Our data support a model in which esterase 6 acts as a direct odorant degrading enzyme for many bioactive food esters, but not cis-vaccenyl acetate.
Organizational Affiliation:
CSIRO Land and Water, Black Mountain, Canberra, ACT, 2601, Australia.