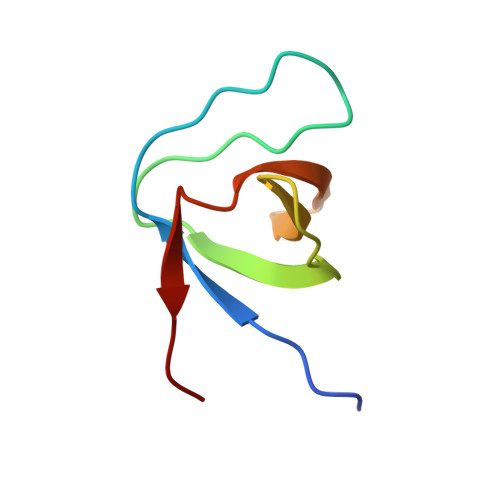
Molecular basis of interactions between SH3 domain-containing proteins and the proline-rich region of the ubiquitin ligase Itch.
Desrochers, G., Cappadocia, L., Lussier-Price, M., Ton, A.T., Ayoubi, R., Serohijos, A., Omichinski, J.G., Angers, A.(2017) J Biol Chem 292: 6325-6338
- PubMed: 28235806
- DOI: https://doi.org/10.1074/jbc.M116.754440
- Primary Citation of Related Structures:
5SXP - PubMed Abstract:
The ligase Itch plays major roles in signaling pathways by inducing ubiquitylation-dependent degradation of several substrates. Substrate recognition and binding are critical for the regulation of this reaction. Like closely related ligases, Itch can interact with proteins containing a PP X Y motif via its WW domains. In addition to these WW domains, Itch possesses a proline-rich region (PRR) that has been shown to interact with several Src homology 3 (SH3) domain-containing proteins. We have previously established that despite the apparent surface uniformity and conserved fold of SH3 domains, they display different binding mechanisms and affinities for their interaction with the PRR of Itch. Here, we attempt to determine the molecular bases underlying the wide range of binding properties of the Itch PRR. Using pulldown assays combined with mass spectrometry analysis, we show that the Itch PRR preferentially forms complexes with endophilins, amphyphisins, and pacsins but can also target a variety of other SH3 domain-containing proteins. In addition, we map the binding sites of these proteins using a combination of PRR sub-sequences and mutants. We find that different SH3 domains target distinct proline-rich sequences overlapping significantly. We also structurally analyze these protein complexes using crystallography and molecular modeling. These structures depict the position of Itch PRR engaged in a 1:2 protein complex with β-PIX and a 1:1 complex with the other SH3 domain-containing proteins. Taken together, these results reveal the binding preferences of the Itch PRR toward its most common SH3 domain-containing partners and demonstrate that the PRR region is sufficient for binding.
Organizational Affiliation:
From the Departments of Biological Sciences and.