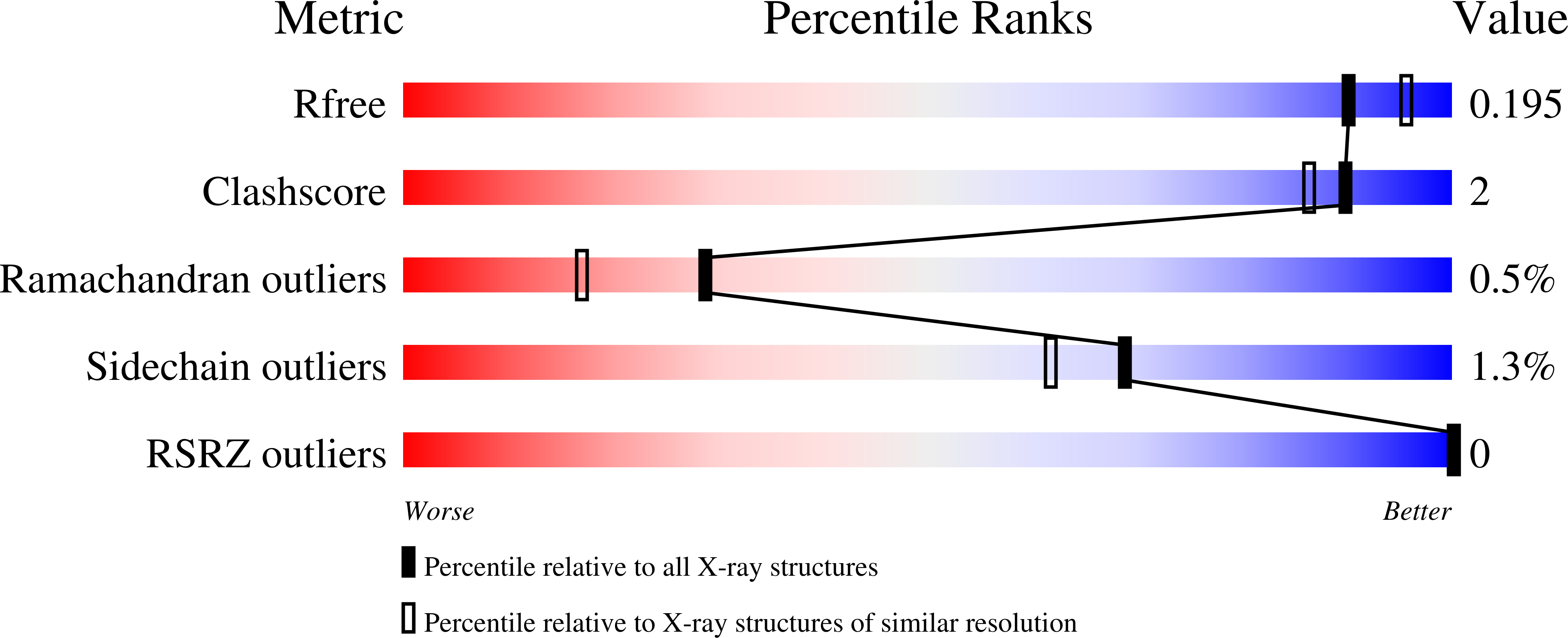
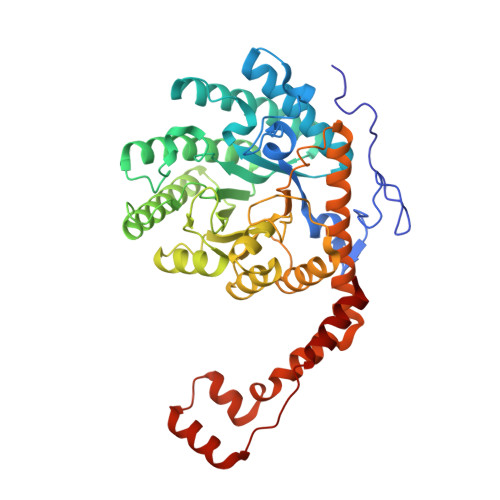
Metal Dependence of the Xylose Isomerase from Piromyces sp. E2 Explored by Activity Profiling and Protein Crystallography.
Lee, M., Rozeboom, H.J., de Waal, P.P., de Jong, R.M., Dudek, H.M., Janssen, D.B.(2017) Biochemistry 56: 5991-6005
- PubMed: 29045784
- DOI: https://doi.org/10.1021/acs.biochem.7b00777
- Primary Citation of Related Structures:
5NH4, 5NH5, 5NH6, 5NH7, 5NH8, 5NH9, 5NHA, 5NHB, 5NHC, 5NHD, 5NHE, 5NHM - PubMed Abstract:
Xylose isomerase from Piromyces sp. E2 (PirXI) can be used to equip Saccharomyces cerevisiae with the capacity to ferment xylose to ethanol. The biochemical properties and structure of the enzyme have not been described even though its metal content, catalytic parameters, and expression level are critical for rapid xylose utilization. We have isolated the enzyme after high-level expression in Escherichia coli, analyzed the metal dependence of its catalytic properties, and determined 12 crystal structures in the presence of different metals, substrates, and substrate analogues. The activity assays revealed that various bivalent metals can activate PirXI for xylose isomerization. Among these metals, Mn 2+ is the most favorable for catalytic activity. Furthermore, the enzyme shows the highest affinity for Mn 2+ , which was established by measuring the activation constants (K act ) for different metals. Metal analysis of the purified enzyme showed that in vivo the enzyme binds a mixture of metals that is determined by metal availability as well as affinity, indicating that the native metal composition can influence activity. The crystal structures show the presence of an active site similar to that of other xylose isomerases, with a d-xylose binding site containing two tryptophans and a catalytic histidine, as well as two metal binding sites that are formed by carboxylate groups of conserved aspartates and glutamates. The binding positions and conformations of the metal-coordinating residues varied slightly for different metals, which is hypothesized to contribute to the observed metal dependence of the isomerase activity.
Organizational Affiliation:
Biochemical Laboratory, Groningen Biomolecular Sciences and Biotechnology Institute, University of Groningen , Nijenborgh 4, 9747 AG Groningen, The Netherlands.