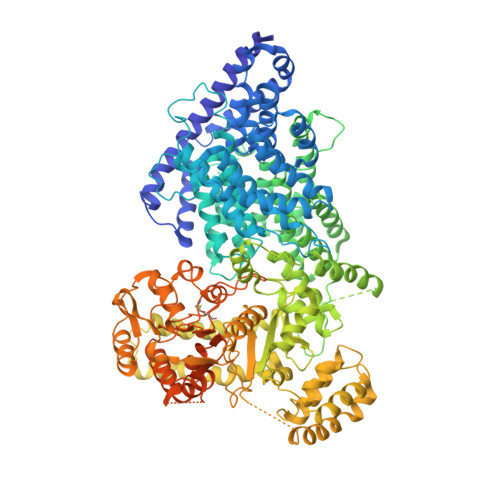
Cryo-EM structure of a metazoan separase-securin complex at near-atomic resolution.
Boland, A., Martin, T.G., Zhang, Z., Yang, J., Bai, X.C., Chang, L., Scheres, S.H., Barford, D.(2017) Nat Struct Mol Biol 24: 414-418
- PubMed: 28263324
- DOI: https://doi.org/10.1038/nsmb.3386
- Primary Citation of Related Structures:
5MZ6 - PubMed Abstract:
Separase is a caspase-family protease that initiates chromatid segregation by cleaving the kleisin subunits (Scc1 and Rec8) of cohesin, and regulates centrosome duplication and mitotic spindle function through cleavage of kendrin and Slk19. To understand the mechanisms of securin regulation of separase, we used single-particle cryo-electron microscopy (cryo-EM) to determine a near-atomic-resolution structure of the Caenorhabditis elegans separase-securin complex. Separase adopts a triangular-shaped bilobal architecture comprising an N-terminal tetratricopeptide repeat (TPR)-like α-solenoid domain docked onto the conserved C-terminal protease domain. Securin engages separase in an extended antiparallel conformation, interacting with both lobes. It inhibits separase by interacting with the catalytic site through a pseudosubstrate mechanism, thus revealing that in the inhibited separase-securin complex, the catalytic site adopts a conformation compatible with substrate binding. Securin is protected from cleavage because an aliphatic side chain at the P1 position represses protease activity by disrupting the organization of catalytic site residues.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Cambridge, UK.