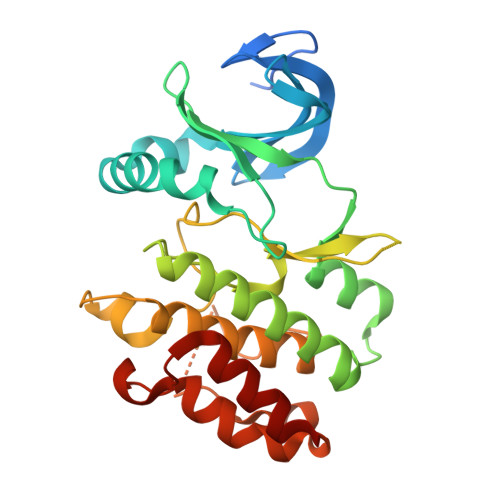
The crystal structure of PknI from Mycobacterium tuberculosis shows an inactive, pseudokinase-like conformation.
Lisa, M.N., Wagner, T., Alexandre, M., Barilone, N., Raynal, B., Alzari, P.M., Bellinzoni, M.(2017) FEBS J 284: 602-614
- PubMed: 28054744
- DOI: https://doi.org/10.1111/febs.14003
- Primary Citation of Related Structures:
5M06, 5M07, 5M08, 5M09 - PubMed Abstract:
Eukaryotic-like Ser/Thr protein kinases (ePKs) have been identified in many bacterial species, where they are known to mediate signalling mechanisms that share several features with their eukaryotic counterparts. In Mycobacterium tuberculosis, PknI is one of the 11 predicted ePKs and it has been related to bacterial virulence. In order to better understand the molecular basis of its role in mycobacterial signalling, we solved the crystal structure of the PknI cytoplasmic domain. We found that even though PknI possesses most conserved elements characteristic of Hanks-type kinases, it is degraded in several motifs that are essential for the ePKs catalytic activity. Most notably, PknI presents a remarkably short activation segment lacking a peptide-substrate binding site. Consistent with this observation and similar to earlier findings for eukaryotic pseudokinases, no kinase activity was detected for the catalytic domain of PknI, against different substrates and in various experimental conditions. Based on these results, we conclude that PknI may rely on unconventional mechanism(s) for kinase activity and/or it could play alternative role(s) in mycobacterial signalling. Atomic coordinates and structure factors for the catalytic domain of M. tuberculosis PknI are in the Protein Data Bank under the accession codes 5M06 (wild-type PknI + ADP), 5M07 (PknI_C20A), 5M08 (PknI_C20A_R136A) and 5M09 (PknI_C20A_R136N).
Organizational Affiliation:
Unité de Microbiologie Structurale, Institut Pasteur, Paris Cedex, France.