Differential lipid binding of vinculin isoforms promotes quasi-equivalent dimerization.
Chinthalapudi, K., Rangarajan, E.S., Brown, D.T., Izard, T.(2016) Proc Natl Acad Sci U S A 113: 9539-9544
- PubMed: 27503891
- DOI: https://doi.org/10.1073/pnas.1600702113
- Primary Citation of Related Structures:
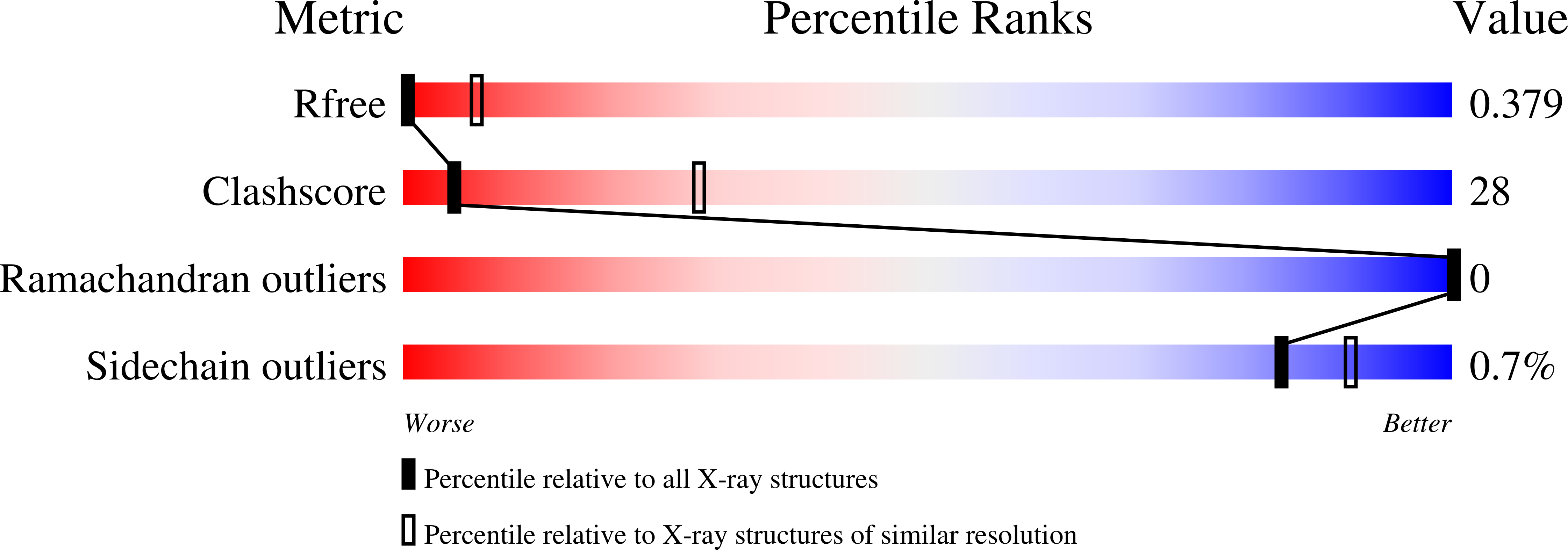
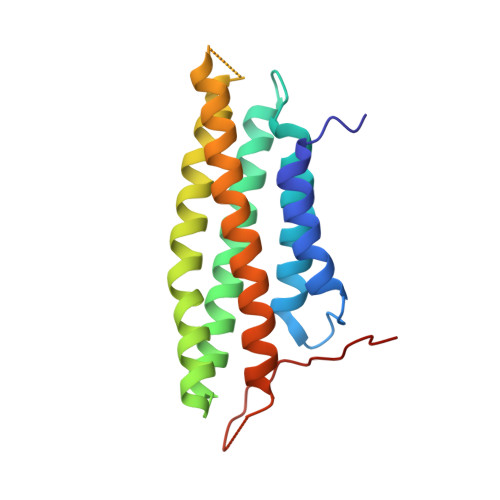
5L0C, 5L0D, 5L0F, 5L0G, 5L0H, 5L0I, 5L0J - PubMed Abstract:
The main cause of death globally remains debilitating heart conditions, such as dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM), which are often due to mutations of specific components of adhesion complexes. Vinculin regulates these complexes and plays essential roles in intercalated discs that are necessary for muscle cell function and coordinated movement and in the development and function of the heart. Humans bearing familial or sporadic mutations in vinculin suffer from chronic, progressively debilitating DCM that ultimately leads to cardiac failure and death, whereas autosomal dominant mutations in vinculin can also provoke HCM, causing acute cardiac failure. The DCM/HCM-associated mutants of vinculin occur in the 68-residue insert unique to the muscle-specific, alternatively spliced isoform of vinculin, termed metavinculin (MV). Contrary to studies that suggested that phosphoinositol-4,5-bisphosphate (PIP2) only induces vinculin homodimers, which are asymmetric, we show that phospholipid binding results in a domain-swapped symmetric MV dimer via a quasi-equivalent interface compared with vinculin involving R975. Although one of the two PIP2 binding sites is preserved, the symmetric MV dimer that bridges two PIP2 molecules differs from the asymmetric vinculin dimer that bridges only one PIP2 Unlike vinculin, wild-type MV and the DCM/HCM-associated R975W mutant bind PIP2 in their inactive conformations, and R975W MV fails to dimerize. Mutating selective vinculin residues to their corresponding MV residues, or vice versa, switches the isoform's dimeric constellation and lipid binding site. Collectively, our data suggest that MV homodimerization modulates microfilament attachment at muscular adhesion sites and furthers our understanding of MV-mediated cardiac remodeling.
Organizational Affiliation:
Cell Adhesion Laboratory, Department of Cancer Biology, The Scripps Research Institute, Jupiter, FL 33458; Department of Immunology and Microbial Sciences, The Scripps Research Institute, Jupiter, FL 33458;