Crystal structure of the signaling protein complex 2
Kumar, V., van den Akker, F.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Alr2278 protein | 182 | Nostoc sp. PCC 7120 = FACHB-418 | Mutation(s): 1 Gene Names: alr2278 | 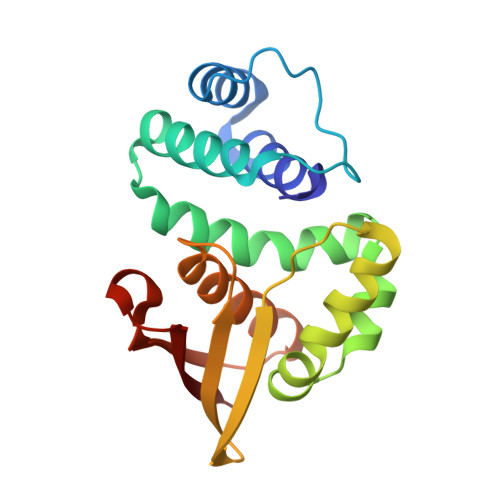 | |
UniProt | |||||
Find proteins for Q8YUQ7 (Nostoc sp. (strain PCC 7120 / SAG 25.82 / UTEX 2576)) Explore Q8YUQ7 Go to UniProtKB: Q8YUQ7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8YUQ7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| HEM Query on HEM | C [auth A], F [auth B] | PROTOPORPHYRIN IX CONTAINING FE C34 H32 Fe N4 O4 KABFMIBPWCXCRK-RGGAHWMASA-L |  | ||
| MLI Query on MLI | E [auth A], H [auth B] | MALONATE ION C3 H2 O4 OFOBLEOULBTSOW-UHFFFAOYSA-L |  | ||
| 1MZ Query on 1MZ | D [auth A], G [auth B] | 1-METHYLIMIDAZOLE C4 H7 N2 MCTWTZJPVLRJOU-UHFFFAOYSA-O |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 122.387 | α = 90 |
| b = 122.387 | β = 90 |
| c = 122.387 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI) | United States | R01 HL075329 |