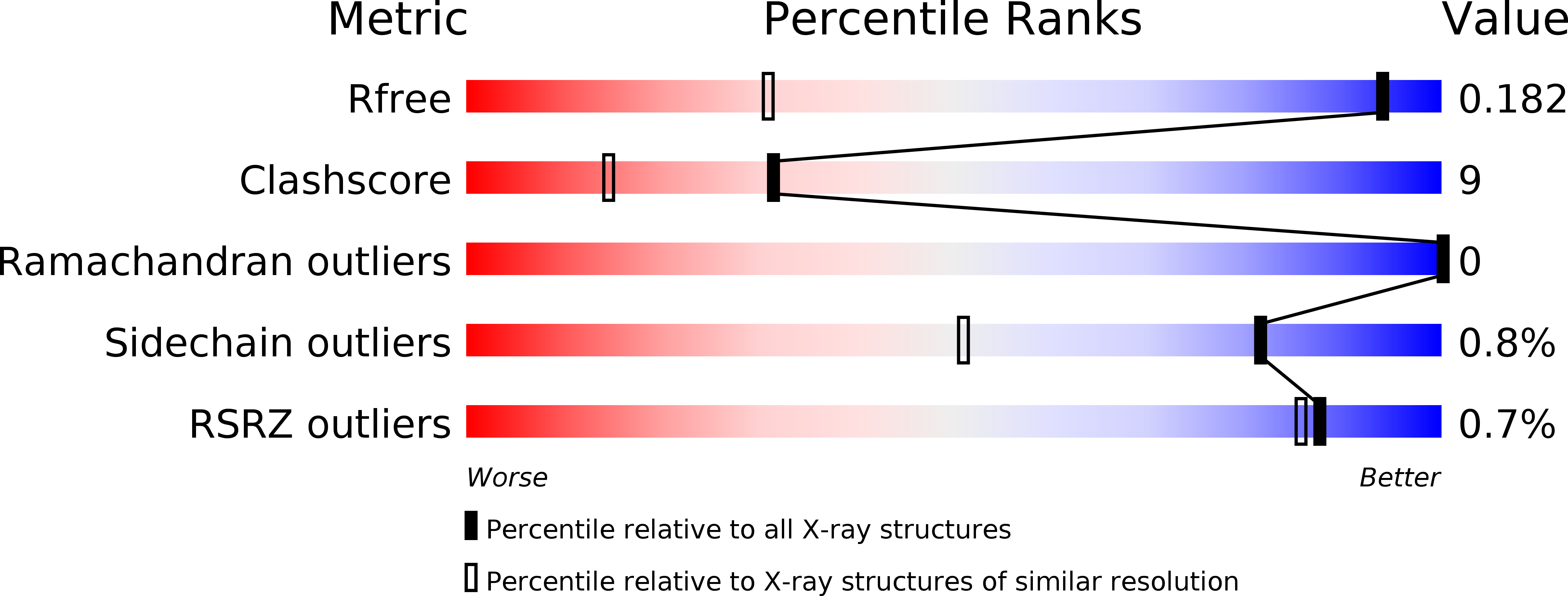
Entry History & Funding Information
Deposition Data
- Released Date: 2017-03-29
Deposition Author(s): Noresson, A.-L., Aurelius, O., Oberg, C.T., Engstrom, O., Sundin, A.P., Hakansson, M., Logan, D.T., Leffler, H., Nilsson, U.J.
| Funding Organization | Location | Grant Number |
|---|
| Swedish Research Council | Sweden | 621-2009-5326 |
| Swedish Research Council | Sweden | 621-2012-2978 |
| Olle Engkvist Byggmastare foundation | Sweden | -- |
| Royal Physiographic Society | Sweden | -- |
| European Community's Seventh Framework Program (FP7-2007-2013) | | HEALTH F2-2011-256986 project acronym PANACREAS |
- Version 1.0: 2017-03-29
Type: Initial release
- Version 1.1: 2018-01-17
Changes: Data collection - Version 1.2: 2024-01-10
Changes: Data collection, Database references, Refinement description