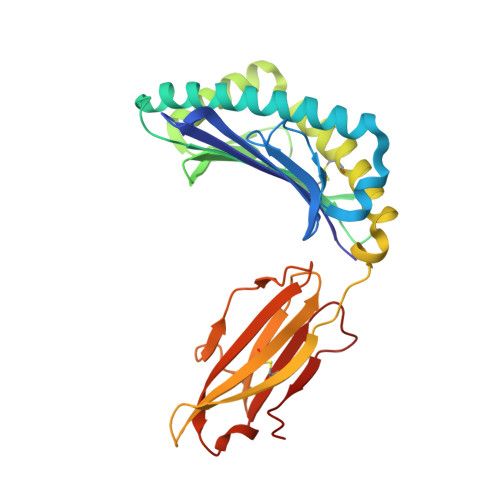
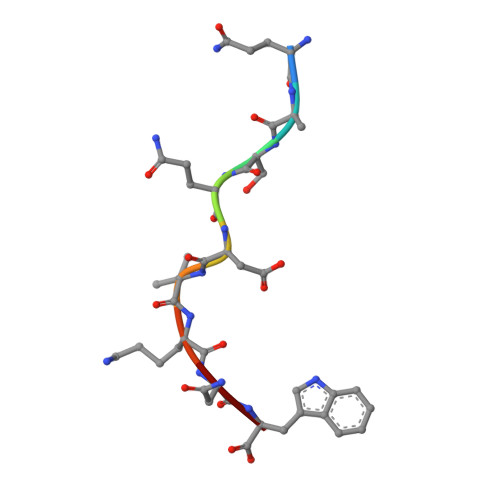
Crystal structure of HLA-B*5801, a protective HLA allele for HIV-1 infection.
Li, X., Lamothe, P.A., Ng, R., Xu, S., Teng, M., Walker, B.D., Wang, J.H.(2016) Protein Cell 7: 761-765
- PubMed: 27638468
- DOI: https://doi.org/10.1007/s13238-016-0309-y
- Primary Citation of Related Structures:
5IM7, 5INC, 5IND
Organizational Affiliation:
School of Life Sciences, University of Science and Technology of China, Hefei, 230027, China.