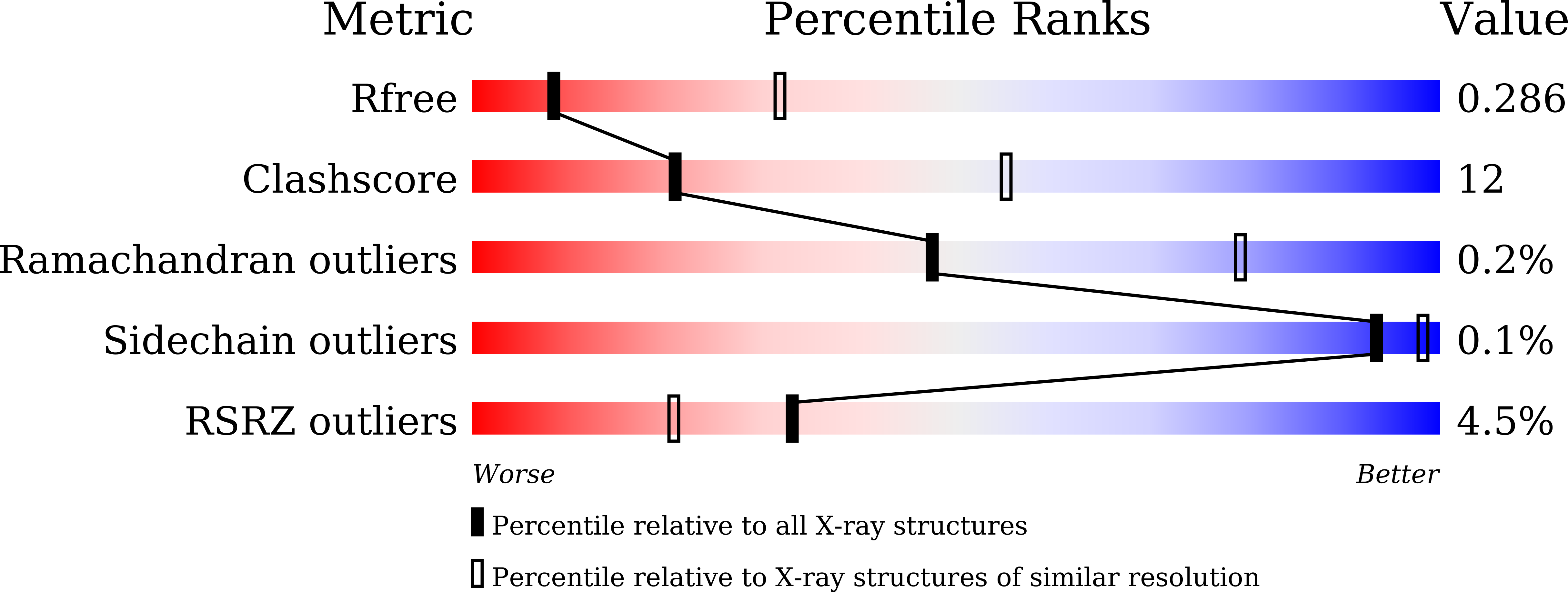
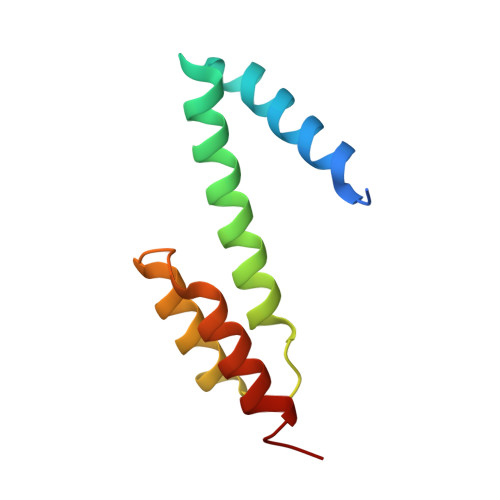
Construction of novel repeat proteins with rigid and predictable structures using a shared helix method.
Youn, S.J., Kwon, N.Y., Lee, J.H., Kim, J.H., Choi, J., Lee, H., Lee, J.O.(2017) Sci Rep 7: 2595-2595
- PubMed: 28572639
- DOI: https://doi.org/10.1038/s41598-017-02803-z
- Primary Citation of Related Structures:
5H75, 5H76, 5H77, 5H78, 5H79, 5H7A, 5H7B, 5H7C, 5H7D, 5X3F, 5XBY - PubMed Abstract:
Generating artificial protein assemblies with complex shapes requires a method for connecting protein components with stable and predictable structures. Currently available methods for creating rigid protein assemblies rely on either complicated calculations or extensive trial and error. We describe a simple and efficient method for connecting two proteins via a fused alpha helix that is formed by joining two preexisting helices into a single extended helix. Because the end-to-end ligation of helices does not guarantee the formation of a continuous helix, we superimposed 1-2 turns of pairs of connecting helices by using a molecular graphics program. Then, we chose amino acids from the two natural sequences that would stabilize the connecting helix. This "shared helix method" is highly efficient. All the designed proteins that could be produced in Escherichia coli were readily crystallized and had the expected fusion structures. To prove the usefulness of this method, we produced two novel repeat proteins by assembling several copies of natural or artificial proteins with alpha helices at both termini. Their crystal structures demonstrated the successful assembly of the repeating units with the intended curved shapes. We propose that this method could dramatically expand the available repertoire of natural repeat proteins.
Organizational Affiliation:
Department of Chemistry, Korea Advanced Institute of Science and Technology, Daejeon, 34141, Korea.