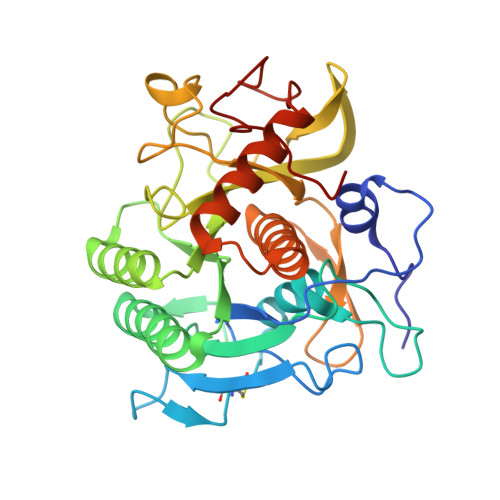
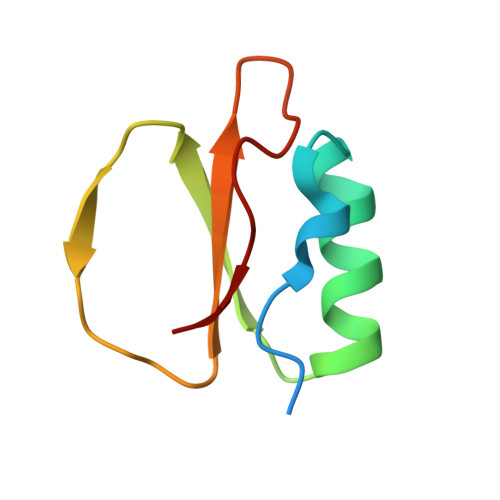
Stabilization of Enzymes by Metal Binding: Structures of Two Alkalophilic Bacillus Subtilases and Analysis of the Second Metal-Binding Site of the Subtilase Family
Dohnalek, J., McAuley, K.E., Brzozowski, A.M., Oestergaard, P.R., Svendsen, A., Wilson, K.S.(2016) Book : 203-266