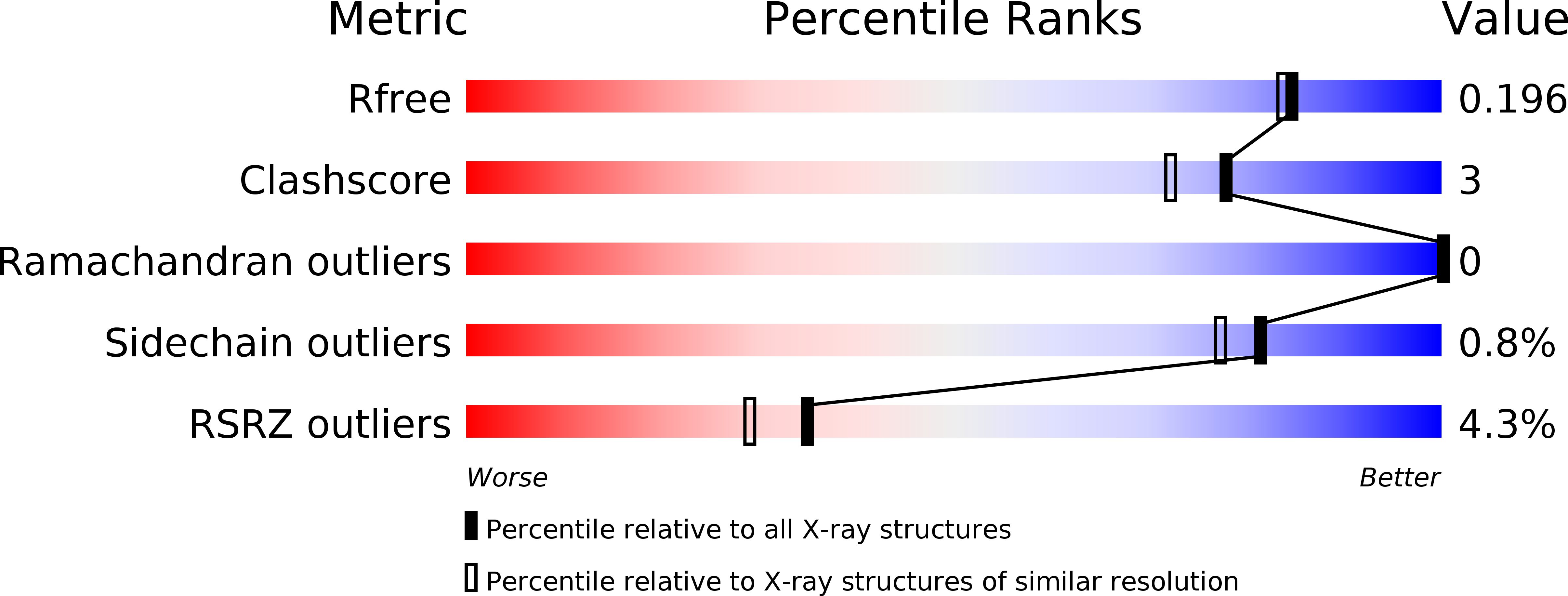
A novel esterase subfamily with alpha / beta-hydrolase fold suggested by structures of two bacterial enzymes homologous to l-homoserine O-acetyl transferases.
Tolzer, C., Pal, S., Watzlawick, H., Altenbuchner, J., Niefind, K.(2016) FEBS Lett 590: 174-184
- PubMed: 26787467
- DOI: https://doi.org/10.1002/1873-3468.12031
- Primary Citation of Related Structures:
5D6O, 5D7B, 5E4Y, 5EFZ - PubMed Abstract:
MekB from Pseudomonas veronii and CgHle from Corynebacteriumglutamicum belong to the superfamily of α/β-hydrolase fold proteins. Based on sequence comparisons, they are annotated as homoserine transacetylases in popular databases like UNIPROT, PFAM or ESTHER. However, experimentally, MekB and CgHle were shown to be esterases that hydrolyse preferentially acetic acid esters. We describe the x-ray structures of these enzymes solved to high resolution. The overall structures confirm the close relatedness to experimentally validated homoserine acetyl transferases, but simultaneously the structures exclude the ability of MekB and CgHle to bind homoserine and acetyl-CoA. Insofar the MekB and CgHle structures suggest dividing the homoserine transacetylase family into subfamilies, namely genuine acetyl transferases and acetyl esterases with MekB and CgHle as constituting members of the latter.
Organizational Affiliation:
Department für Chemie, Institut für Biochemie, Universität zu Köln, Germany.